Targeted Polymerase Chain Reaction-Based Enrichment and Next Generation Sequencing for Diagnostic Testing of Congenital Disorders of Glycosylation Melanie A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seq2pathway Vignette
seq2pathway Vignette Bin Wang, Xinan Holly Yang, Arjun Kinstlick May 19, 2021 Contents 1 Abstract 1 2 Package Installation 2 3 runseq2pathway 2 4 Two main functions 3 4.1 seq2gene . .3 4.1.1 seq2gene flowchart . .3 4.1.2 runseq2gene inputs/parameters . .5 4.1.3 runseq2gene outputs . .8 4.2 gene2pathway . 10 4.2.1 gene2pathway flowchart . 11 4.2.2 gene2pathway test inputs/parameters . 11 4.2.3 gene2pathway test outputs . 12 5 Examples 13 5.1 ChIP-seq data analysis . 13 5.1.1 Map ChIP-seq enriched peaks to genes using runseq2gene .................... 13 5.1.2 Discover enriched GO terms using gene2pathway_test with gene scores . 15 5.1.3 Discover enriched GO terms using Fisher's Exact test without gene scores . 17 5.1.4 Add description for genes . 20 5.2 RNA-seq data analysis . 20 6 R environment session 23 1 Abstract Seq2pathway is a novel computational tool to analyze functional gene-sets (including signaling pathways) using variable next-generation sequencing data[1]. Integral to this tool are the \seq2gene" and \gene2pathway" components in series that infer a quantitative pathway-level profile for each sample. The seq2gene function assigns phenotype-associated significance of genomic regions to gene-level scores, where the significance could be p-values of SNPs or point mutations, protein-binding affinity, or transcriptional expression level. The seq2gene function has the feasibility to assign non-exon regions to a range of neighboring genes besides the nearest one, thus facilitating the study of functional non-coding elements[2]. Then the gene2pathway summarizes gene-level measurements to pathway-level scores, comparing the quantity of significance for gene members within a pathway with those outside a pathway. -

Autism Multiplex Family with 16P11.2P12.2 Microduplication Syndrome in Monozygotic Twins and Distal 16P11.2 Deletion in Their Brother
European Journal of Human Genetics (2012) 20, 540–546 & 2012 Macmillan Publishers Limited All rights reserved 1018-4813/12 www.nature.com/ejhg ARTICLE Autism multiplex family with 16p11.2p12.2 microduplication syndrome in monozygotic twins and distal 16p11.2 deletion in their brother Anne-Claude Tabet1,2,3,4, Marion Pilorge2,3,4, Richard Delorme5,6,Fre´de´rique Amsellem5,6, Jean-Marc Pinard7, Marion Leboyer6,8,9, Alain Verloes10, Brigitte Benzacken1,11,12 and Catalina Betancur*,2,3,4 The pericentromeric region of chromosome 16p is rich in segmental duplications that predispose to rearrangements through non-allelic homologous recombination. Several recurrent copy number variations have been described recently in chromosome 16p. 16p11.2 rearrangements (29.5–30.1 Mb) are associated with autism, intellectual disability (ID) and other neurodevelopmental disorders. Another recognizable but less common microdeletion syndrome in 16p11.2p12.2 (21.4 to 28.5–30.1 Mb) has been described in six individuals with ID, whereas apparently reciprocal duplications, studied by standard cytogenetic and fluorescence in situ hybridization techniques, have been reported in three patients with autism spectrum disorders. Here, we report a multiplex family with three boys affected with autism, including two monozygotic twins carrying a de novo 16p11.2p12.2 duplication of 8.95 Mb (21.28–30.23 Mb) characterized by single-nucleotide polymorphism array, encompassing both the 16p11.2 and 16p11.2p12.2 regions. The twins exhibited autism, severe ID, and dysmorphic features, including a triangular face, deep-set eyes, large and prominent nasal bridge, and tall, slender build. The eldest brother presented with autism, mild ID, early-onset obesity and normal craniofacial features, and carried a smaller, overlapping 16p11.2 microdeletion of 847 kb (28.40–29.25 Mb), inherited from his apparently healthy father. -

Linkage Analysis in 15 Families, Physical And
European Journal of Human Genetics (2002) 11, 145 – 154 ª 2002 Nature Publishing Group All rights reserved 1018 – 4813/02 $25.00 www.nature.com/ejhg ARTICLE Familial juvenile hyperuricaemic nephropathy (FJHN): linkage analysis in 15 families, physical and transcriptional characterisation of the FJHN critical region on chromosome 16p11.2 and the analysis of seven candidate genes Blanka Stibu˚ rkova´1, Jacek Majewski2, Katerˇina Hodanˇova´1, Lenka Ondrova´1, Marke´ta Jerˇa´bkova´1, Marie Zika´nova´1, Petr Vylet’al1, Ivan Sˇebesta3, Anthony Marinaki4, Anne Simmonds4, Gert Matthijs5, Jean-Pierre Fryns5, Rosa Torres6, Juan Garcı´a Puig7, Jurg Ott2 and Stanislav Kmoch*,1 1Center for Integrated Genomics, Institute for Inherited Metabolic Disorders, Charles University 1st School of Medicine and General Faculty Hospital Prague, Czech Republic; 2Laboratory of Statistical Genetics, Rockefeller University, New York, NY, USA; 3Department of Clinical Biochemistry, Charles University 1st School of Medicine and General Faculty Hospital Prague, Czech Republic; 4Purine Research Unit, GKT, Guy’s Hospital, London, UK; 5Center for Human Genetics, University of Leuven, Leuven, Belgium; 6Department of Clinical Biochemistry, La Paz Hospital, Madrid, Spain; 7Division of Internal Medicine, La Paz Hospital, Madrid, Spain Familial juvenile hyperuricaemic nephropathy (FJHN) is an autosomal dominant renal disease characterised by juvenile onset of hyperuricaemia, gouty arthritis, and progressive renal failure at an early age. Recent studies in four kindreds showed linkage of a gene for FJHN to the same genomic interval on chromosome 16p11.2, where the gene for the phenotypically similar medullary cystic disease type 2 (MCKD2) has been localised. In this study we performed linkage analysis in additional 15 FJHN families. -

Congenital Disorders of Glycosylation from a Neurological Perspective
brain sciences Review Congenital Disorders of Glycosylation from a Neurological Perspective Justyna Paprocka 1,* , Aleksandra Jezela-Stanek 2 , Anna Tylki-Szyma´nska 3 and Stephanie Grunewald 4 1 Department of Pediatric Neurology, Faculty of Medical Science in Katowice, Medical University of Silesia, 40-752 Katowice, Poland 2 Department of Genetics and Clinical Immunology, National Institute of Tuberculosis and Lung Diseases, 01-138 Warsaw, Poland; [email protected] 3 Department of Pediatrics, Nutrition and Metabolic Diseases, The Children’s Memorial Health Institute, W 04-730 Warsaw, Poland; [email protected] 4 NIHR Biomedical Research Center (BRC), Metabolic Unit, Great Ormond Street Hospital and Institute of Child Health, University College London, London SE1 9RT, UK; [email protected] * Correspondence: [email protected]; Tel.: +48-606-415-888 Abstract: Most plasma proteins, cell membrane proteins and other proteins are glycoproteins with sugar chains attached to the polypeptide-glycans. Glycosylation is the main element of the post- translational transformation of most human proteins. Since glycosylation processes are necessary for many different biological processes, patients present a diverse spectrum of phenotypes and severity of symptoms. The most frequently observed neurological symptoms in congenital disorders of glycosylation (CDG) are: epilepsy, intellectual disability, myopathies, neuropathies and stroke-like episodes. Epilepsy is seen in many CDG subtypes and particularly present in the case of mutations -

Genome-Wide Copy Number Variant Analysis For
An et al. BMC Medical Genomics (2016) 9:2 DOI 10.1186/s12920-015-0163-4 RESEARCH ARTICLE Open Access Genome-wide copy number variant analysis for congenital ventricular septal defects in Chinese Han population Yu An1,2,4, Wenyuan Duan3, Guoying Huang4, Xiaoli Chen5,LiLi5, Chenxia Nie6, Jia Hou4, Yonghao Gui4, Yiming Wu1, Feng Zhang2, Yiping Shen7, Bailin Wu1,4,7* and Hongyan Wang8* Abstract Background: Ventricular septal defects (VSDs) constitute the most prevalent congenital heart disease (CHD), occurs either in isolation (isolated VSD) or in combination with other cardiac defects (complex VSD). Copy number variation (CNV) has been highlighted as a possible contributing factor to the etiology of many congenital diseases. However, little is known concerning the involvement of CNVs in either isolated or complex VSDs. Methods: We analyzed 154 unrelated Chinese individuals with VSD by chromosomal microarray analysis. The subjects were recruited from four hospitals across China. Each case underwent clinical assessment to define the type of VSD, either isolated or complex VSD. CNVs detected were categorized into syndrom related CNVs, recurrent CNVs and rare CNVs. Genes encompassed by the CNVs were analyzed using enrichment and pathway analysis. Results: Among 154 probands, we identified 29 rare CNVs in 26 VSD patients (16.9 %, 26/154) and 8 syndrome-related CNVs in 8 VSD patients (5.2 %, 8/154). 12 of the detected 29 rare CNVs (41.3 %) were recurrently reported in DECIPHER or ISCA database as associated with either VSD or general heart disease. Fifteen genes (5 %, 15/285) within CNVs were associated with a broad spectrum of complicated CHD. -

Prevalence and Incidence of Rare Diseases: Bibliographic Data
Number 1 | January 2019 Prevalence and incidence of rare diseases: Bibliographic data Prevalence, incidence or number of published cases listed by diseases (in alphabetical order) www.orpha.net www.orphadata.org If a range of national data is available, the average is Methodology calculated to estimate the worldwide or European prevalence or incidence. When a range of data sources is available, the most Orphanet carries out a systematic survey of literature in recent data source that meets a certain number of quality order to estimate the prevalence and incidence of rare criteria is favoured (registries, meta-analyses, diseases. This study aims to collect new data regarding population-based studies, large cohorts studies). point prevalence, birth prevalence and incidence, and to update already published data according to new For congenital diseases, the prevalence is estimated, so scientific studies or other available data. that: Prevalence = birth prevalence x (patient life This data is presented in the following reports published expectancy/general population life expectancy). biannually: When only incidence data is documented, the prevalence is estimated when possible, so that : • Prevalence, incidence or number of published cases listed by diseases (in alphabetical order); Prevalence = incidence x disease mean duration. • Diseases listed by decreasing prevalence, incidence When neither prevalence nor incidence data is available, or number of published cases; which is the case for very rare diseases, the number of cases or families documented in the medical literature is Data collection provided. A number of different sources are used : Limitations of the study • Registries (RARECARE, EUROCAT, etc) ; The prevalence and incidence data presented in this report are only estimations and cannot be considered to • National/international health institutes and agencies be absolutely correct. -

MECHANISMS in ENDOCRINOLOGY: Novel Genetic Causes of Short Stature
J M Wit and others Genetics of short stature 174:4 R145–R173 Review MECHANISMS IN ENDOCRINOLOGY Novel genetic causes of short stature 1 1 2 2 Jan M Wit , Wilma Oostdijk , Monique Losekoot , Hermine A van Duyvenvoorde , Correspondence Claudia A L Ruivenkamp2 and Sarina G Kant2 should be addressed to J M Wit Departments of 1Paediatrics and 2Clinical Genetics, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, Email The Netherlands [email protected] Abstract The fast technological development, particularly single nucleotide polymorphism array, array-comparative genomic hybridization, and whole exome sequencing, has led to the discovery of many novel genetic causes of growth failure. In this review we discuss a selection of these, according to a diagnostic classification centred on the epiphyseal growth plate. We successively discuss disorders in hormone signalling, paracrine factors, matrix molecules, intracellular pathways, and fundamental cellular processes, followed by chromosomal aberrations including copy number variants (CNVs) and imprinting disorders associated with short stature. Many novel causes of GH deficiency (GHD) as part of combined pituitary hormone deficiency have been uncovered. The most frequent genetic causes of isolated GHD are GH1 and GHRHR defects, but several novel causes have recently been found, such as GHSR, RNPC3, and IFT172 mutations. Besides well-defined causes of GH insensitivity (GHR, STAT5B, IGFALS, IGF1 defects), disorders of NFkB signalling, STAT3 and IGF2 have recently been discovered. Heterozygous IGF1R defects are a relatively frequent cause of prenatal and postnatal growth retardation. TRHA mutations cause a syndromic form of short stature with elevated T3/T4 ratio. Disorders of signalling of various paracrine factors (FGFs, BMPs, WNTs, PTHrP/IHH, and CNP/NPR2) or genetic defects affecting cartilage extracellular matrix usually cause disproportionate short stature. -
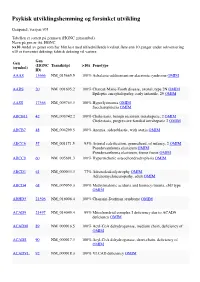
Psykisk Utviklingshemming Og Forsinket Utvikling
Psykisk utviklingshemming og forsinket utvikling Genpanel, versjon v03 Tabellen er sortert på gennavn (HGNC gensymbol) Navn på gen er iht. HGNC >x10 Andel av genet som har blitt lest med tilfredstillende kvalitet flere enn 10 ganger under sekvensering x10 er forventet dekning; faktisk dekning vil variere. Gen Gen (HGNC Transkript >10x Fenotype (symbol) ID) AAAS 13666 NM_015665.5 100% Achalasia-addisonianism-alacrimia syndrome OMIM AARS 20 NM_001605.2 100% Charcot-Marie-Tooth disease, axonal, type 2N OMIM Epileptic encephalopathy, early infantile, 29 OMIM AASS 17366 NM_005763.3 100% Hyperlysinemia OMIM Saccharopinuria OMIM ABCB11 42 NM_003742.2 100% Cholestasis, benign recurrent intrahepatic, 2 OMIM Cholestasis, progressive familial intrahepatic 2 OMIM ABCB7 48 NM_004299.5 100% Anemia, sideroblastic, with ataxia OMIM ABCC6 57 NM_001171.5 93% Arterial calcification, generalized, of infancy, 2 OMIM Pseudoxanthoma elasticum OMIM Pseudoxanthoma elasticum, forme fruste OMIM ABCC9 60 NM_005691.3 100% Hypertrichotic osteochondrodysplasia OMIM ABCD1 61 NM_000033.3 77% Adrenoleukodystrophy OMIM Adrenomyeloneuropathy, adult OMIM ABCD4 68 NM_005050.3 100% Methylmalonic aciduria and homocystinuria, cblJ type OMIM ABHD5 21396 NM_016006.4 100% Chanarin-Dorfman syndrome OMIM ACAD9 21497 NM_014049.4 99% Mitochondrial complex I deficiency due to ACAD9 deficiency OMIM ACADM 89 NM_000016.5 100% Acyl-CoA dehydrogenase, medium chain, deficiency of OMIM ACADS 90 NM_000017.3 100% Acyl-CoA dehydrogenase, short-chain, deficiency of OMIM ACADVL 92 NM_000018.3 100% VLCAD -

DPAGT1 Antibody (Pab)
21.10.2014DPAGT1 antibody (pAb) Rabbit Anti-Human/Mouse DPAGT1 (DGPT, GPT, ALG7, G1PT, UAGT, UGAT, CDG1J, CDG -Ij) Instruction Manual Catalog Number PK-AB718-6485 Synonyms DPAGT1 Antibody: DPAGT, DGPT, GPT, ALG7, G1PT, UAGT, UGAT, CDG1J, CDG-Ij, UDP-N- acetylglucosamine-dolichyl-phosphate N-acetylglucosaminephosphotransferase Description The UDP-N-acetylglucosamine-dolichyl-phosphate N-acetyl-glucosaminephosphotransferase (DPAGT1) is an enzyme that catalyzes the first step in the dolichol-linked oligosaccharide pathway for glycoprotein biosynthesis. Mutations in this integral endoplasmic reticulum (ER) membrane protein enzyme belongs to the glycosyltransferase family 4 results in the congenital disorder of glycosylation type Ij with symptoms such as severe hypotonia, medically intractable seizures, mental retardation, microcephaly, and exotropia. Recent experiments have shown that DPAGT1 is a target of the Wnt/beta-catenin signaling pathway, with Wnt3a inducing higher DPAGT1 mRNA expression. Quantity 100 µg Source / Host Rabbit Immunogen DPAGT1 antibody was raised against a 17 amino acid synthetic peptide near the amino terminus of human DPAGT1. Purification Method Affinity chromatography purified via peptide column. Clone / IgG Subtype Polyclonal antibody Species Reactivity Human, Mouse Specificity At least four isoforms of DPAGT1 are known to exist; this antibody will recognize the two longest isoforms. DPAGT1 antibody is predicted to not cross-react with UHRF1BP1. Formulation Antibody is supplied in PBS containing 0.02% sodium azide. Reconstitution During shipment, small volumes of antibody will occasionally become entrapped in the seal of the product vial. For products with volumes of 200 μl or less, we recommend gently tapping the vial on a hard surface or briefly centrifuging the vial in a tabletop centrifuge to dislodge any liquid in the container’s cap. -

Impacts of Massively Parallel Sequencing for Genetic Diagnosis of Neuromuscular Disorders
Acta Neuropathol (2013) 125:173–185 DOI 10.1007/s00401-012-1072-7 REVIEW Impacts of massively parallel sequencing for genetic diagnosis of neuromuscular disorders Nasim Vasli • Jocelyn Laporte Received: 8 October 2012 / Revised: 27 November 2012 / Accepted: 28 November 2012 / Published online: 7 December 2012 Ó Springer-Verlag Berlin Heidelberg 2012 Abstract Neuromuscular disorders (NMD) such as neu- We remind the challenges and benefit of obtaining an ropathy or myopathy are rare and often severe inherited accurate genetic diagnosis, introduce the massively parallel disorders, affecting muscle and/or nerves with neonatal, sequencing technology and its novel applications in diag- childhood or adulthood onset, with considerable burden for nosis of patients, prenatal diagnosis and carrier detection, the patients, their families and public health systems. and discuss the limitations and necessary improvements. Genetic and clinical heterogeneity, unspecific clinical fea- Massively parallel sequencing synergizes with clinical and tures, unidentified genes and the implication of large and/or pathological investigations into an integrated diagnosis several genes requiring complementary methods are the approach. Clinicians and pathologists are crucial in patient main drawbacks in routine molecular diagnosis, leading to selection and interpretation of data, and persons trained in increased turnaround time and delay in the molecular data management and analysis need to be integrated to the validation of the diagnosis. The application of massively diagnosis pipeline. Massively parallel sequencing for parallel sequencing, also called next generation sequenc- mutation identification is expected to greatly improve ing, as a routine diagnostic strategy could lead to a rapid diagnosis, genetic counseling and patient management. screening and fast identification of mutations in rare genetic disorders like NMD. -

Sphingomyelin Suppresses Hedgehog Signaling by Restricting Cholesterol Accessibility at the Ciliary Membrane
bioRxiv preprint doi: https://doi.org/10.1101/699819; this version posted July 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Sphingomyelin suppresses Hedgehog signaling by restricting cholesterol accessibility at the ciliary membrane 1# 1# 1 1 1 Maia Kinnebrew , Ellen J. Iverson , Bhaven B. Patel , Ganesh V. Pusapati , Jennifer H. Kong , Kristen A. 3 1,5 4 2 3* Johnson , Giovanni Luchetti , Douglas F. Covey , Christian Siebold , Arun Radhakrishnan and Rajat Rohatgi1* 1 Departments of Biochemistry and Medicine, Stanford University School of Medicine, Stanford, California, United States of America 2 Division of Structural Biology, Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK 3 Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX 75390 4 Department of Developmental Biology and Taylor Family Institute for Innovative Psychiatric Research, Washington University School of Medicine, St. Louis, Missouri, United States of America 5 Current address: Department of Physiological Chemistry, Genentech, South San Francisco, CA # MK and EJI contributed equally to this study. * Correspondence to [email protected] or [email protected] bioRxiv preprint doi: https://doi.org/10.1101/699819; this version posted July 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. -

Open Full Page
Research Article The Majority of Viral-Cellular Fusion Transcripts in Cervical Carcinomas Cotranscribe Cellular Sequences of Known or Predicted Genes Irene Kraus,1,3,4 Corina Driesch,4 Svetlana Vinokurova,5 Eivind Hovig,2 AchimSchneider, 6 Magnus von Knebel Doeberitz,5 and Matthias Du¨rst4 1Institute of Pathology, Rikshospitalet University Hospital; 2Department of Tumor Biology, Institute for Cancer Research, The Norwegian Radium Hospital, Oslo, Norway; 3NorChip AS, Klokkarstua, Norway; 4Gyna¨kologischeMolekularbiologie, Frauenklinik der Friedrich-Schiller-Universita¨t,Jena, Germany; 5Department of Applied Tumor Biology, Institute of Pathology, University of Heidelberg, Heidelberg, Germany; and 6Frauenklinik mit Hochschulambulanz der Charite´, Campus Benjamin Franklin und Campus Mitte, Berlin, Germany Abstract virus lies in its oncogenes E6 and E7, underlined by their con- Integration of human papillomavirus (HPV) DNA into the host stitutive expression in HPV-induced cervical carcinomas (4, 5). The genome is a frequent event in cervical carcinogenesis and is E6 and E7 oncoproteins mediate mitogenic and antiapoptotic reported to occur at randomly selected chromosomal sites. stimuli by interacting with numerous regulatory proteins of the host cell that control the cell cycle (6, 7). Moreover, the viral However,as the databases are being up-dated continuously, the knowledge based on sequenced viral integration sites also oncoproteins induce mitotic defects and genomic instability by expands. In this study,viral-cellular fusion transcripts of a uncoupling centrosome duplication from the cell division cycle preselected group of 74 cervical carcinoma or cervical (8, 9). During malignant progression, viral DNA is frequently intraepithelial neoplasia grade 3 (CIN3) biopsies harboring integrated into the host genome (10, 11).