3-27-2017 Nerve Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chlorpyrifos (Dursban) Ddvp (Dichlorvos) Diazinon Malathion Parathion
CHLORPYRIFOS (DURSBAN) DDVP (DICHLORVOS) DIAZINON MALATHION PARATHION Method no.: 62 Matrix: Air Procedure: Samples are collected by drawing known volumes of air through specially constructed glass sampling tubes, each containing a glass fiber filter and two sections of XAD-2 adsorbent. Samples are desorbed with toluene and analyzed by GC using a flame photometric detector (FPD). Recommended air volume and sampling rate: 480 L at 1.0 L/min except for Malathion 60 L at 1.0 L/min for Malathion Target concentrations: 1.0 mg/m3 (0.111 ppm) for Dichlorvos (PEL) 0.1 mg/m3 (0.008 ppm) for Diazinon (TLV) 0.2 mg/m3 (0.014 ppm) for Chlorpyrifos (TLV) 15.0 mg/m3 (1.11 ppm) for Malathion (PEL) 0.1 mg/m3 (0.008 ppm) for Parathion (PEL) Reliable quantitation limits: 0.0019 mg/m3 (0.21 ppb) for Dichlorvos (based on the RAV) 0.0030 mg/m3 (0.24 ppb) for Diazinon 0.0033 mg/m3 (0.23 ppb) for Chlorpyrifos 0.0303 mg/m3 (2.2 ppb) for Malathion 0.0031 mg/m3 (0.26 ppb) for Parathion Standard errors of estimate at the target concentration: 5.3% for Dichlorvos (Section 4.6.) 5.3% for Diazinon 5.3% for Chlorpyrifos 5.6% for Malathion 5.3% for Parathion Status of method: Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. Date: October 1986 Chemist: Donald Burright Organic Methods Evaluation Branch OSHA Analytical Laboratory Salt Lake City, Utah 1 of 27 T-62-FV-01-8610-M 1. -

Hawaii on Two Foliage Plants, Dwarf Brassaia Diazinon Plant After Spots
in Japan and England will be given by Tosh Fu- {Brassaia arboricola) and Dwarf Ti {Cordyline chikami of O.M. Scotts and Ray McMicken of B. terminalis 'Madameandre') to determine their Hayman Co., respectively. phototoxicity to selected insecticides and acara- cides. Plants, growing in 6-inch pots, were treated Farwest Show by submerging the aerial portions of the plant in Farwest Nursery, Garden, and Supply Show water suspensions of 7 pesticides for 15seconds. will be September 8-10, 1975, at the Memorial Granular formulations of 2 pesticides were ap Coliseum in Portland, Oregon. For information plied to the soil surface. Materials, at 2X stand contact: Farwest Nursery Show, Suite GA-7, ard rates, were as follows: 222 S.W. Harrison, Protland, OR. 97201. Amount formulation per: ASHS The 72d annual meeting of ASHS (American Material and formulation 1-gallon water 6-inch pot Society for Horticultural Science) will be held in chlorobenzilate 4E 2t — Honolulu, September 8 to 13, 1975, at the dicofol (Kelthane) 35WP 2T - Sheraton-Waikiki Hotel. Meeting concurrently Pentac 50WP 2T — with ASHS will be the American Horticulture carbaryl (Sevin) 50WP 2T - Society. The University of Hawaii will host the diazinon AG500 (48% EC) 2t - meeting with Dr. Richard Bullock, general chair dimethoate (Cygon) 2E 2t — man. Dr. Henry Nakasone will serve as assistant Volck Oil Supreme 2T - general chairman, Dr. Phil Parvin as local arrange aldicarb (Temik) 10G - 1.5t ments chairman, and Dr. Richard Criley as pro disulfoton (Di-Syston) 15G - 1.5t gram chairman. Neighbor island tours will be untreated controls - conducted following the meetings. -

Azinphos Methyl Analysis of Risks to Endangered and Threatened Salmon and Steelhead
Azinphos methyl Analysis of Risks to Endangered and Threatened Salmon and Steelhead July 23, 2003 William Erickson, Ph.D. and Larry Turner, Ph.D. Environmental Field Branch Office of Pesticide Programs Summary Azinphos methyl is an organophosphate pesticide registered for control of insects on a variety of crops, mainly fruits and nuts. Azinphos methyl is very highly toxic to fish and aquatic invertebrates. An ecological risk assessment that includes nontarget aquatic organisms was prepared by OPP’s Environmental Fate and Effects Division (EFED) in 1999, and an Interim Reregistration Eligibility Decision (IRED) was issued in October of 2001. The assessment concludes that acute and chronic Levels of Concern (LOCs) are exceeded for threatened and endangered (T&E or listed) freshwater fish as a result of runoff and drift of azinphos methyl from all treatment sites. Acute and chronic levels of concern also are exceeded for individuals of T&E invertebrates, as well as populations of invertebrates that may serve as food for listed fish. A subsequent agreement between azinphos methyl registrants and the Agency has led to 23 uses being canceled; seven other uses being phased out in 2005. Mitigation measures will reduce application rates and add no-spray buffers to product labels. Despite these measures, we conclude that azinphos methyl may affect 25 Evolutionarily Significant Units (ESUs) and will have no effect on one ESU. Our determinations are based on the known or potential use of azinphos methyl on various use sites in each county where there is habitat or a migration corridor for an ESU and the acute and chronic risks of azinphos methyl to endangered fish. -

Diazinon 50W INSECTICIDE
RESTRICTED USE PESTICIDE DUE TO AVIAN AND AQUATIC TOXICITY FOR RETAIL SALE TO AND USE ONLY BY CERTIFIED APPLICATORS OR PERSONS UNDER THEIR DIRECT SUPERVISION AND ONLY FOR THOSE USES COVERED BY THE CERTIFIED APPLICATOR’S CERTIFICATION. Diazinon 50W INSECTICIDE For control of certain insects on fruits, vegetables, nuts, field crops, and ornamentals grown outdoors in nurseries. ACTIVE INGREDIENT % BY WT. Diazinon: O,O-diethyl O-(2-isopropyl-6-methyl-4-pyrimidinyl) phosphorothioate ................................................................................................. 50.0% INERT INGREDIENTS ....................................................................................................................................................................................................... 50.0% TOTAL................................................................................................................................................................................................................................. 100.0% EPA Reg. No. 66222-10 KEEP OUT OF REACH OF CHILDREN CAUTION FIRST AID CONTAINS AN ORGANOPHOSPHATE THAT INHIBITS CHOLINESTERASE IF SWALLOWED: Call a poison control center or doctor immediately for treatment advice. Do not induce vomiting unless told to by a poison con- trol center or doctor. Have person sip a glass of water if able to swallow. Do not give anything by mouth to an unconscious or convulsing person. IF INHALED: Move person to fresh air. If person is not breathing, call 911 or an ambulance, then -

Innovative Biocatalysts As Tools to Detect and Inactivate Nerve Agents Elena Porzio1, Francesca Bettazzi2, Luigi Mandrich1, Immacolata Del Giudice1, Odile F
www.nature.com/scientificreports OPEN Innovative Biocatalysts as Tools to Detect and Inactivate Nerve Agents Elena Porzio1, Francesca Bettazzi2, Luigi Mandrich1, Immacolata Del Giudice1, Odile F. Restaino3, Serena Laschi4, Ferdinando Febbraio1, Valentina De Luca1, 3 1 5 6 Received: 24 February 2018 Maria G. Borzacchiello , Teresa M. Carusone , Franz Worek , Antonio Pisanti , Piero Porcaro6, Chiara Schiraldi3, Mario De Rosa3, Ilaria Palchetti 2 & Giuseppe Manco1 Accepted: 25 July 2018 Published: xx xx xxxx Pesticides and warfare nerve agents are frequently organophosphates (OPs) or related compounds. Their acute toxicity highlighted more than ever the need to explore applicable strategies for the sensing, decontamination and/or detoxifcation of these compounds. Herein, we report the use of two diferent thermostable enzyme families capable to detect and inactivate OPs. In particular, mutants of carboxylesterase-2 from Alicyclobacillus acidocaldarius and of phosphotriesterase-like lactonases from Sulfolobus solfataricus and Sulfolobus acidocaldarius, have been selected and assembled in an optimized format for the development of an electrochemical biosensor and a decontamination formulation, respectively. The features of the developed tools have been tested in an ad-hoc fabricated chamber, to mimic an alarming situation of exposure to a nerve agent. Choosing ethyl-paraoxon as nerve agent simulant, a limit of detection (LOD) of 0.4 nM, after 5 s of exposure time was obtained. Furthermore, an optimized enzymatic formulation was used for a fast and efcient environmental detoxifcation (>99%) of the nebulized nerve agent simulants in the air and on surfaces. Crucial, large- scale experiments have been possible thanks to production of grams amounts of pure (>90%) enzymes. Pesticides and warfare nerve agents are frequently organophosphates (OPs) or related compounds (e.g. -

MATERIAL SAFETY DATA SHEET Diazinon AG500 Page 1 of 6
MATERIAL SAFETY DATA SHEET Diazinon AG500 Page 1 of 6 1. IDENTIFICATION Product name: Diazinon AG500 (EPA Reg. No. 66222-9) Chemical name of active ingredient(s): Diazinon: O,O-diethyl O-(2-isopropyl-6-methyl-4-pyrimidinyl) phosphorothioate Manufacturer/Registrant: Makhteshim Agan of North America, Inc. 3120 Highwoods Blvd., Suite 100 Raleigh, NC 27604 Phone: 919-256-9300 For fire, spill, and/or leak emergencies, contact Phone: 1-800-535-5053 Infotrac: For medical emergencies and health and Phone: 1-877-250-9291 safety inquiries, contact Prosar: 2. COMPOSITION/INFORMATION ON INGREDIENTS COMMON NAME CAS NO. % OSHA PEL ACGIH TLV OTHER NTP/IARC/OSHA (CARCINOGEN) Diazinon 333-41-5 48 NE 0.1 mg/m3 (TWA) NE NA Aromatic petroleum 123 mg/m3 hydrocarbons 25551-13-7 39.2 NE (TWA) NE NA Contains: Pseudocumene (1,2,4- 123 mg/m3 trimethylbenzene) 95-63-6 9.0* NE (TWA) NE NA 435 mg/m3 434 mg/m3 m-Xylene 108-38-3 3.1* (TWA) (TWA) NE NA 435 mg/m3 434 mg/m3 o-Xylene 95-47-6 3.1* (TWA) (TWA) NE NA *Amount in finished product. NE=not established; NA=not available 3. HAZARDS IDENTIFICATIONS PHYSICAL PROPERTIES: Appearance: Amber liquid Odor: Slight sulfur-like EMERGENCY OVERVIEW: CAUTION. Harmful if swallowed, inhaled or absorbed through skin. Causes moderate eye injury. Avoid contact with eyes, skin, or clothing. Avoid breathing vapor or spray mist. Prolonged or frequently repeated skin contact may cause allergic reactions in some individuals. Avoid contamination of food and feed. Food utensils such as tablespoons and measuring cups should not be used for food purposes after use in measuring pesticides. -

Qsar Analysis of the Chemical Hydrolysis of Organophosphorus Pesticides in Natural Waters
QSAR ANALYSIS OF THE CHEMICAL HYDROLYSIS OF ORGANOPHOSPHORUS PESTICIDES IN NATURAL WATERS. by Kenneth K. Tanji Principal Investigator and Jonathan 1. Sullivan Graduate Research Assistant Department of Land, Air and Water Resources University of California, Davis Technical Completion Report Project Number W-843 August, 1995 University of California Water Resource Center The research leading to this report was supported by the University of California Water Resource Center as part of Water Resource Center Project W-843. Table of Contents Page Abstract 2 Problem and Research Objectives 3 Introduction 5 Theoretical Background 6 QSAR Methodology 7 Molecular Connectivity Theory 8 Organophosphorus Pesticides 12 Experimental Determination of Rates 15 Results and Discussion 17 Principal Findings and Significance 19 References 34 List of Tables Page Table 1. Statistical relationship between OP pesticides and first-order MC/'s. 30 Table 2. Inherent conditions of waters used in experimental work. 16 Table 3. Estimated half-lives for organophosphorus esters derived from model. 31 Table 4. Half-lives and first-order MCI' sfor model calibration data set. 31 Table 5. Experimental kinetic data for validation set compounds, Sacramento. 33 List of Figures Page Figure 1. Essential Features OfQSAR Modeling Methodology. 21 Figure 2. Regression plot for In hydrolysis rate vs. 1st order MCl' s. 22 Figure 3. a 3-D molecular model, a line-segment model and a graphical model. 23 Figure 4. Molecular connectivity index suborders. 24 Figure 5. Chlorpyrifos and its fourteen fourth order path/cluster fragments. 25 Figure 6. Abridged MClndex output. 26 Figure 7. Parent acids of most common organophosphorus pesticides. 12 Figure 8. -

Carbaryl Human Health and Ecological Risk Assessment Revised Final Report
SERA TR-052-01-05a Carbaryl Human Health and Ecological Risk Assessment Revised Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0009 SERA Internal Task No. 52-01 Submitted by: Patrick R. Durkin and Cynthia King Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com February 9, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables .................................................................................................................................. v List of Attachments........................................................................................................................ vi List of Appendices ......................................................................................................................... vi COMMON UNIT CONVERSIONS AND ABBREVIATIONS................................................... ix CONVERSION OF SCIENTIFIC NOTATION ............................................................................ x EXECUTIVE SUMMARY .......................................................................................................... -

United States Patent (19) 11 Patent Number: 5,703,064 Yokoi Et Al
US005703064A United States Patent (19) 11 Patent Number: 5,703,064 Yokoi et al. 45) Date of Patent: Dec. 30, 1997 54 PESTICIDAL COMBINATIONS FOREIGN PATENT DOCUMENTS 75 Inventors: Shinji Yokoi; Akira Nishida, both of 0 196524 10/1986 European Pat. Of.. Shiga-ken; Tokio Obata; Kouichi Golka, both of Ube, all of Japan OTHER PUBLICATIONS 73) Assignees: Sankyo Company, Limited, Tokyo; Worthing et al, The Pesticide Manual, 9th Ed. (1991), pp. Ube industries Ltd., Ube, both of 747 and 748. Japan L.C. Gaughan et al., "Pesticide interactions: effects of orga nophosphorus pesticides on the metabolism, toxicity, and 21 Appl. No.: 405,795 persistence of selected pyrethroid insecticides". Chemical Abstracts, vol. 94, No. 9, 1981, No. 59740k of Pestic. 22 Filed: Mar 16, 1995 Biochem. Physio., vol. 14, No. 1, 1980, pp. 81-85. 30 Foreign Application Priority Data I. Ishaaya et al., "Cypermethrin synergism by pyrethroid esterase inhibitors in adults of the whitefly Bemisia tabaci". Mar 16, 1994 JP Japan ............................ HE6045.405 Chemical Abstracts, vol. 107, No. 9, 1987, No. 72818y of (51) Int. Cl................. A01N 43/54; A01N 57/00 Pestic Biochem. Physiol., vol. 28, No. 2, 1987, pp. 155-162. 52 U.S. C. ......................................... 51480; 514/256 (58) Field of Search ..................................... 514/80, 256 Primary Examiner Allen J. Robinson Attorney, Agent, or Firm-Frishauf, Holtz, Goodman, 56 References Cited Langer & Chick, P.C. U.S. PATENT DOCUMENTS 57 ABSTRACT 4,374,833 2/1983 Badmin et al. ...................... 424/225 Combinations of the known compound pyrimidifen with 4,845,097 7/1989 Matsumoto et al... 514/234.2 phosphorus-containing pesticides have a synergistic pesti 4,935,516 6/1990 Ataka et al. -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -
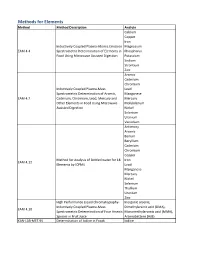
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

Insecticide Resistance Management for Codling Moth Control
Orchard IPM: Insecticide Resistance Management for Codling Moth Control Insecticide resistance trials at the Penn State effective in controlling mid-summer woolly apple aphid Fruit Research and Extension Center, along (WAA) in most Pennsylvania apple orchards and if eventually with recent apple load rejections due to internal eliminated by the US-EPA, it will not be that greatly missed in worms, indicate a need to re-focus attention on eastern apple orchards. It remains a critical product for strategies to slow down the development of controlling peach tree borers and the American plum borer in cherry with its only effective replacement, Thionex, being insecticide resistance in codling moth. already eliminated because it belonged in the same pesticide class as DDT. Diazinon is limited to only a single application each year for only WAA. The Reduced-Risk products that replaced them such as Confirm, Intrepid, Calypso, Provado, Actara, and Avaunt were much safer to people and the environment, although the neonicotinoid insecticides are now often portrayed erroneously by the public as the main cause of bee declines. Limitations for Managing Pesticide Resistance The bar by which insecticides are now measured has changed in the last 15 years so that human safety is not enough, and safety for the environment, and especially bees, is required for Codling moth larvae. Photo: David Biddinger, Penn State new pesticide registrations. Because of this and the merging of 25 pesticide companies 15 years ago into only five or six, the Changes in Product Registrations development of new insecticides for specialty crops has slowed to a crawl.