Biological Safety Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biohazardous Waste Treatment
Biological Safety: Principles & Applications for Lab Personnel Presented by: Biological Safety Office http://biosafety.utk.edu Introduction OVERVIEW OF BIOSAFETY PROGRAM Introduction to Laboratory Safety When entering the laboratory environment you are likely to contact hazards that you would not encounter on a daily basis. These can include: • Physical hazards • Chemical hazards • Radiological hazards • Biological hazards Biohazards Any biological agent or condition that poses a threat to human, animal, or plant health, or to the environment. Examples include, but not limited to: • Agents causing disease in humans, animals or plants (bacteria, viruses, etc.) • Toxins of biological origin • Materials potentially containing infectious agents or biohazards .Blood, tissues, body fluids etc. .Waste, carcasses etc. • Recombinant DNA (depends) • Nanoparticles w/biological effector conjugates (depends) Types of Biohazards: What they are.... How they’ll look.... Regulations & Standards The Institutional Biosafety Committee Institutional Biosafety Committees (IBC) are required by the National Institutes of Health Office of Biological Activities (NIH; OBA) for institutions that receive NIH funding and conduct research using recombinant or synthetic nucleic acids. The UT IBC is composed of 14 UT-affiliated members and 2 non-affiliated members, who collectively offer a broad range of experience in research, safety and public health. Functions of the IBC Projects requiring IBC review • Performing both initial and annual • Those involving recombinant -

Biological Safety Standard Operating Procedure (SOP) SOP Title
IBSP005 Biosafety SOP template January 2021 Icahn School of Medicine at Mount Sinai Biological Safety Standard Operating Procedure (SOP) SOP Title SOP Number (version) IBC registration(s) Section 1. Laboratory-specific information Building/Room(s) Department/Institute SOP author LSO PI name PI signature Section 2. Biological hazard information Biological Agent1 RG2 BSL3 Potential Hazards Signs/Symptoms of Occupational Health ABSL4 Infection Requirements Section 3. Other hazards Sharps Hazards Other Biological Hazards Chemical Hazards Radiological Hazards Section 4. Personal Protective Equipment (PPE) Laboratory Coat Fluid-Resistant Gown Surgical Mask Gloves Shoe Covers N95 Respirator Eye Protection Fluid-Resistant Sleeves PAPR Other PPE 1 Specific strains of biological agents should be described in the corresponding IBC registration. 2 NIH Risk Groups: RG-1, RG-2, RG-3, or RG-4 3 Biological Safety Level: BSL-1, BSL-2, BSL-3, BSL-4 4 Animal Biological Safety Level: ABSL-1, ABSL-2, ABSL-3, ABSL-4 Page | 1 IBSP005 Biosafety SOP template January 2021 Section 5. Equipment and Engineering Controls Biological Safety Cabinet Centrifuge Aerosol-Generating Equipment Other equipment Section 6. Decontamination Disinfectant Contact Time Dilution Location Section 7. Waste Management/Disposal Steam Sterilizer Location: (Autoclave) Chemical Disinfectant(s) Location: Other Disinfectant(s) Location: Section 8. Transport Procedure(s) Page | 2 IBSP005 Biosafety SOP template January 2021 Section 9. Spill Response Procedure Section 10. Protocol Procedure Page | 3 IBSP005 Biosafety SOP template January 2021 Section 10. Protocol Procedure Continued (attach additional sheets if necessary) Page | 4 IBSP005 Biosafety SOP template January 2021 Section 11. Documentation of training and understanding. The Principal Investigator must ensure that all laboratory personnel receive training on the content of this SOP. -

Occupational Safety and Health Admin., Labor § 1910.145
Occupational Safety and Health Admin., Labor § 1910.145 of the workers or their families, shall (i) Fire protection equipment and appa- be provided in connection with all food ratus. [Reserved] handling facilities. There shall be no (ii) Danger. Safety cans or other port- direct opening from living or sleeping able containers of flammable liquids quarters into a kitchen or dining hall. having a flash point at or below 80° F, (3) No person with any communicable table containers of flammable liquids disease shall be employed or permitted (open cup tester), excluding shipping to work in the preparation, cooking, containers, shall be painted red with serving, or other handling of food, food- some additional clearly visible identi- stuffs, or materials used therein, in fication either in the form of a yellow any kitchen or dining room operated in band around the can or the name of the connection with a camp or regularly contents conspicuously stenciled or used by persons living in a camp. painted on the can in yellow. Red (j) Insect and rodent control. Effective lights shall be provided at barricades measures shall be taken to prevent in- and at temporary obstructions, as spec- festation by and harborage of animal ified in ANSI Safety Code for Building or insect vectors or pests. Construction, A10.2±1944, which is in- (k) First aid. (1) Adequate first aid fa- corporated by reference as specified in cilities approved by a health authority § 1910.6. Danger signs shall be painted shall be maintained and made available red. in every labor camp for the emergency (iii) Stop. -

Field Trials for GM Food Get Green Light in India
NATURE|Vol 448|23 August 2007 NEWS IN BRIEF Field trials for GM food Asthmatics win payment in diesel-fumes lawsuit get green light in India Asthma patients in Tokyo last week welcomed a cash settlement India’s first genetically modified (GM) food from car manufacturers and crop is a step closer to reaching the dining the Japanese government. The table. The government has approved field one-time payment resolved a trials for a strain of brinjal (aubergine) decade-long legal battle in which carrying a Bt (Bacillus thuringiensis) gene. the asthmatics blamed diesel car Jalna-based Mahyco, the Indian venture fumes for their illness. KIM KYUNG-HOON/REUTERS of US seed giant Monsanto, claims its insect- The automakers, including resistant variety gives better yields with Toyota, Honda and Nissan, will less pesticide use. To avoid possible cross- provide ¥1.2 billion (US$10.5 contamination with farmers’ crops, the trials million) to the plaintiffs, and a will be carried out in government farms. further ¥3.3 billion to support But critics already campaigning against Bt a five-year health plan for the cotton — currently the only GM crop grown patients. The central government in India — say the brinjal trials are illegal. and Tokyo metropolitan Full biosafety data on the brinjal tests government will each contribute ¥6 billion to the medical programme. have not yet been generated, says Kavitha Kuruganti of the Centre for Sustainable scientists — to pay for relocation, housing starting dose “should be considered for Agriculture, based in Hyderabad: “The trials and research. “This is about the rescue of patients with certain genetic variations”. -
Lab Standard Operating Procedures (Sops)
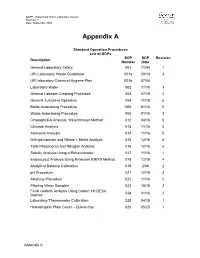
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

Common Personal Protective Equipment for Laboratories
Common Personal Protective Equipment The following document is to provide general guidance for common types of Personal Protective Equipment (PPE) within a laboratory. PPE should be selected based on the hazards present and task performed and is a last line of defense to protect personnel from hazards. All PPE has limitations. If there are any specific questions regarding PPE, please consult your Principal Investigator, the Laboratory Safety Unit, or the University’s Personal Protective Equipment Program. Please contact EH&S with questions regarding PPE selection and hazard assessments. Face and Eye Protection: Must be ANSI Z87.1 compliant Type of PPE: General Uses: Limitations Vapor Goggles • Conforms tight to the • Not designed face for impact • Fully seals around the protection eyes to protect against • Limited splash vapors protection • Protection from heavy particulates (wood/sand/debris) Safety Glasses • Eye protection from • Not designed impact and larger for vapor foreign objects protection • Minimal splash protection Laser Safety • Laser and wavelength • Not designed Glasses/Goggles specific eye protection for vapor or • Must meet current ANSI splash Z136.1 requirements protection and be clearly labeled with optical densities (OD) and wavelength Face Shield • Used for full face splash • Not designed protection for vapor • Available in chin, full protection face, or neck length • Certain types can be used for UV and radiological isotopes protection Environmental Health & Safety 1 of 4 May 2021 Glove Protection: Type of PPE: General Uses: Limitations Nitrile Gloves • Disposable, one-time • May not protect use against • General barrier hydrocarbons protection, allows for and alcohols. Nitrile good dexterity Consult a hand alternatives protection are discussed chart, like here. -
Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents
Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents This First Responders Sampling Guide was funded under U.S. EPA Assistance Agreement No. X6-97109101-1. Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents New England Water Works Association 125 Hopping Brook Road Holliston, MA 01746-1471 November 2008 Acknowledgments This Sampling Guide for First Responders to Drinking Water Contamination Threats and Incidents was originally developed by the New England Water Works Association in collaboration with U.S. EPA. Please refer to pages 75 and 76 for a complete list of acknowledgments. The cover photo is reprinted with permission from the Worcester Telegram & Gazette. IMPORTANT NOTICE The methods and instructions in this guide reflect the U.S. Environmental Protection Agency (EPA) regulations and guidance, in addition to other reference documents. It is recognized that there can be significant differences from state to state regarding public notification, sampling procedures, laboratory safety, and handling, etc. Please check with your state drinking water representative with any questions before First Responders training and preparation is begun. Contents, Sections 1 through 6 1. Introduction 1.1 Background/Purpose ......................................1 1.2 How to Use This Guide ....................................2 2. Overview of Site Characterization .........................3 2.1 Investigating the Site .......................................3 2.2 Who Conducts Site Characterization and Sampling? .................................................5 3. Site Characterization Five-Step Process ...............6 3.1 The Five-Step Process .....................................6 Step 1: Customizing the Site Characterization Plan ........................8 Step 2: Approaching the Site and Doing a Field Safety Screening ........9 Step 3: Characterizing the Site ...................11 Step 4: Collecting Samples ..........................14 Step 5: Exiting the Site .................................16 4. -

Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain
COMMODITY SPECIFIC FOOD SAFETY GUIDELINES FOR THE FRESH TOMATO SUPPLY CHAIN Third Edition | September 2018 Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain Tomato Guidelines, 3rd Edition User’s Note These guidelines provide recommended food safety practices that are intended to minimize the microbiological hazards associated with fresh and fresh-cut tomato products. The intent of drafting this document is to provide currently available information on food safety and handling in a manner consistent with existing applicable regulations, standards and guidelines. The information provided herein is offered in good faith and believed to be reliable, but is made without warranty, express or implied, as to merchantability, fitness for a particular purpose, or any other matter. These recommended guidelines were not designed to apply to any specific operation. It is the responsibility of the user of this document to verify that these guidelines are appropriate for its operation. The publishing trade associations, their members and contributors do not assume any responsibility for compliance with applicable laws and regulations, and recommend that users consult with their own legal and technical advisers to be sure that their own procedures meet with applicable requirements. - 1 - Foreword The North American Tomato Trade Work Group (NATTWG) published in 2006 the first edition of Commodity Specific Food Safety Guidelines for the Fresh Tomato Supply Chain (“Guidelines”). Within two years of publication, several initiatives resulted in significant new learnings about potential risks and control measures at all points in the fresh tomato supply chain. Some of those initiatives include the FDA Tomato Safety Initiative, voluntary efforts by the Florida Tomato Exchange and the California Tomato Farmers to develop USDA-verified audit criteria and programs for tomato production and harvest practices in those states, and several retail and foodservice buyer initiatives to further define tomato safe growing and handling practices. -

A New Level 3 Biosafety and Astrobiology Laboratory in Pieve A
PAN.R.C.- A New Level 3 Biosafety and Astrobiology Laboratory in Pieve a Nievole (PT) Tasselli, D. TS Corporation Srl - Astronomy and Astrophysics Department Via Rugantino, 71, 00169 Roma RM - Italy E-mail:[email protected] Ferrara, G. TS Corporation Srl - Biology Department Via Cavalcanti, 10, 51010 Massa e Cozzile PT - Italy E-mail:[email protected] Ricci, S. TS Corporation Srl - Meteorogical and Climatic Change Department Via Rugantino, 71, 00169 Roma RM - Italy E-mail:[email protected] Bianchi, P. TS Corporation Srl - Geology and Geophysics Department Via Rugantino, 71, 00169 Roma RM - Italy E-mail:[email protected] Abstract We report our proposal for the establishment of a biocontainment and astrobiology laboratory in a strategic area of Pieve a Nievole (PT) at 28 mt above sea level - to face the lack of biological and astrobiological research centers and all the social, economic and cultural consequences that this project implicate. The structure will be built under the Horizon 2020 work program 2018-2020 - European Research Infrastructures (in- cluding e-Infrastructures), and will enable the development of major re- arXiv:1811.05201v1 [astro-ph.IM] 13 Nov 2018 search project. Keyword: astrobiology - biosafety - biosecurity - BSL3 - containment - research center - biological risk - biological agents - new research facility - location: Pieve a Nievole (PT) This paper was prepared with the LATEX 1 1 Introduction project that involves the use of biological agents from second or third group, must The environment that surrounds us is pop- ask permission and wait for the technical ulated by biological agents such as bacte- time to use the currently available facili- ria, viruses or fungi. -

Genetically Modified Tree Policy Technology and Innovation, Sustainability Issued By: Date: 18/06/2021 and Institutional Affairs Code: POL.00.0044 Revision
Title: Genetically Modified Tree Policy Technology and Innovation, Sustainability Issued by: Date: 18/06/2021 and Institutional Affairs Code: POL.00.0044 Revision: Contents 1 PURPOSE ............................................................................................................................................................. 3 2 REFERENCE DOCUMENTS ................................................................................................................................. 4 3 TERMS, DEFINITIONS AND ABBREVIATIONS .................................................................................................. 4 4 GUIDELINES ........................................................................................................................................................ 8 4.1 Principles ......................................................................................................................................................... 8 4.2 Governance and Ethics .................................................................................................................................. 9 4.2.1 Advisory Committee .................................................................................................................................. 9 4.2.1.1 Purpose ..................................................................................................................................................... 9 4.2.1.2 Responsibilities ....................................................................................................................................... -

Biosafety Level 2 Guide University of Illinois at Urbana-Champaign
Biosafety Level 2 Guide University of Illinois at Urbana-Champaign Table of Contents Introduction .................................................................................................................................................. 2 Risk Assessments .......................................................................................................................................... 2 Routes of Exposure ................................................................................................................................... 3 Risk Groups and Biosafety Levels .............................................................................................................. 4 IBC Registrations ....................................................................................................................................... 5 Laboratory Audits...................................................................................................................................... 5 Risk Assessment Scenarios ........................................................................................................................ 5 Training requirements .................................................................................................................................. 7 Introduction to Biosafety .......................................................................................................................... 7 NIH Guidelines Overview ......................................................................................................................... -

Finding Hazards
FINDING HAZARDS OSHA 11 Finding Hazards 1 Osha 11 Finding Hazards 2 FINDING HAZARDS Learning Objectives By the end of this lesson, students will be able to: • Define the term “job hazard” • Identify a variety of health and safety hazards found at typical worksites where young people are employed. • Locate various types of hazards in an actual workplace. Time Needed: 45 Minutes Materials Needed • Flipchart Paper • Markers (5 colors per student group) • PowerPoint Slides: #1: Job Hazards #2: Sample Hazard Map #3: Finding Hazards: Key Points • Appendix A handouts (Optional) Preparing To Teach This Lesson Before you present this lesson: 1. Obtain a flipchart and markers or use a chalkboard and chalk. 2. Locate slides #1-3 on your CD and review them. If necessary, copy onto transparencies. 3. For the Hazard Mapping activity, you will need flipchart paper and a set of five colored markers (black, red, green, blue, orange) for each small group. Detailed Instructor’s Notes A. Introduction: What is a job hazard? (15 minutes) 1. Remind the class that a job hazard is anything at work that can hurt you, either physically or mentally. Explain that some job hazards are very obvious, but others are not. In order to be better prepared to be safe on the job, it is necessary to be able to identify different types of hazards. Tell the class that hazards can be divided into four categories. Write the categories across the top of a piece of flipchart paper and show PowerPoint Slide #1, Job Hazards. • Safety hazards can cause immediate accidents and injuries.