Antidiabetic Action of Bezafibrate in a Large Observational Database
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lipid Lowering Agents, Cognitive Decline, and Dementia: the Three-City Study
Lipid lowering agents, cognitive decline, and dementia: the three-city study. Marie-Laure Ancelin, Isabelle Carrière, Pascale Barberger-Gateau, Sophie Auriacombe, Olivier Rouaud, Spiros Fourlanos, Claudine Berr, Anne-Marie Dupuy, Karen Ritchie To cite this version: Marie-Laure Ancelin, Isabelle Carrière, Pascale Barberger-Gateau, Sophie Auriacombe, Olivier Rouaud, et al.. Lipid lowering agents, cognitive decline, and dementia: the three-city study.: Lipid Lowering Agents and Cognitive Decline. Journal of Alzheimer’s Disease, IOS Press, 2012, 30 (3), pp.629-37. 10.3233/JAD-2012-120064. inserm-00707350 HAL Id: inserm-00707350 https://www.hal.inserm.fr/inserm-00707350 Submitted on 12 Jun 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Lipid lowering agents, cognitive decline, and dementia: the three-city study Marie-Laure Ancelin 1 * , Isabelle Carrière 1 , Pascale Barberger-Gateau 2 , Sophie Auriacombe 2 , Olivier Rouaud 3 , Spiros Fourlanos 4 , Claudine Berr 1 , Anne-Marie Dupuy 1 5 , Karen Ritchie 1 6 1 Neuropsychiatrie : Recherche Epidémiologique -
Statins and Lipid Lowering Agents
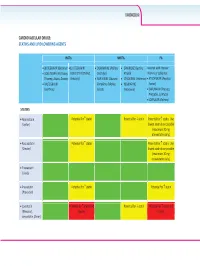
CARDIOVASCULAR Z/Ks^h>ZZh'^͗ ^dd/E^E>/W/>KtZ/E''Ed^ /E^d/Ɛ EEZd/Ɛ W/Ɛ x/d'Zs/Z ;ŝŬƚĂƌǀLJͿ x>s/d'Zs/Zͬ x KZs/Z/E ;WŝĨĞůƚƌŽ͕ x &s/ZE ;^ƵƐƚŝǀĂ͕ ŽŽƐƚĞĚǁŝƚŚƌŝƚŽŶĂǀŝƌ xK>hd'Zs/Z ;dŝǀŝĐĂLJ͕ K//^dd ;^ƚƌŝďŝůĚ͕ ĞůƐƚƌŝŐŽͿ ƚƌŝƉůĂͿ ;EŽƌǀŝƌͿŽƌĐŽďŝĐŝƐƚĂƚ dƌŝƵŵĞƋ͕:ƵůƵĐĂ͕ŽǀĂƚŽͿ 'ĞŶǀŽLJĂͿ x Z/>W/s/Z/E ;ĚƵƌĂŶƚ͕ x dZs/Z/E ;/ŶƚĞůĞŶĐĞͿ xdEs/Z ;ZĞLJĂƚĂnj͕ xZ>d'Zs/Z ŽŵƉůĞƌĂ͕KĚĞĨƐĞLJ͕ x Es/ZW/E ǀŽƚĂnjͿ ;/ƐĞŶƚƌĞƐƐͿ :ƵůƵĐĂͿ ;sŝƌĂŵƵŶĞͿ xZhEs/Z ;WƌĞnjŝƐƚĂ͕ WƌĞnjĐŽďŝdž͕^LJŵƚƵnjĂͿ x>KW/Es/Z ;<ĂůĞƚƌĂͿ ^dd/E^ xƚŽƌǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;>ŝƉŝƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϮϬŵŐ ĂƚŽƌǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xZŽƐƵǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ͘hƐĞ ;ƌĞƐƚŽƌͿ ůŽǁĞƐƚƐƚĂƚŝŶĚŽƐĞƉŽƐƐŝďůĞ ;ŵĂdžŝŵƵŵϭϬŵŐ ƌŽƐƵǀĂƐƚĂƚŝŶĚĂŝůLJͿ͘ xWŝƚĂǀĂƐƚĂƚŝŶ ;>ŝǀĂůŽͿ xWƌĂǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶ ;WƌĂǀĂĐŚŽůͿ x>ŽǀĂƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ WŽƚĞŶƚŝĂůĨŽƌ pƐƚĂƚŝŶ WŽƚĞŶƚŝĂůĨŽƌ nƐƚĂƚŝŶĂŶĚ ;DĞǀĂĐŽƌͿ͕ ƚŽdžŝĐŝƚLJ ƚŽdžŝĐŝƚLJ ƐŝŵǀĂƐƚĂƚŝŶ ;ŽĐŽƌͿ CARDIOVASCULAR INSTIs NNRTIs PIs xBICTEGRAVIR (Biktarvy) xELVITEGRAVIR/ x DORAVIRINE (Pifeltro, x EFAVIRENZ (Sustiva, Boosted with ritonavir xDOLUTEGRAVIR (Tivicay, COBICISTAT (Stribild, Delstrigo) Atripla) (Norvir) or cobicistat Triumeq, Juluca) Genvoya) x RILPIVIRINE (Edurant, x ETRAVIRINE (Intelence) xATAZANAVIR (Reyataz, xRALTEGRAVIR Complera, Odefsey, x NEVIRAPINE Evotaz) (Isentress) Juluca) (Viramune) xDARUNAVIR (Prezista, Prezcobix, Symtuza) xLOPINAVIR (Kaletra) FIBRATES xFenofibrate, bezafibrate, gemfibrozil CHOLESTEROL ABSORPTION INHIBITOR xEzetimibe -

Clinofibrate Improved Canine Lipid Metabolism in Some but Not All Breeds
NOTE Internal Medicine Clinofibrate improved canine lipid metabolism in some but not all breeds Yohtaro SATO1), Nobuaki ARAI2), Hidemi YASUDA3) and Yasushi MIZOGUCHI4)* 1)Graduate School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan 2)Spectrum Lab Japan, 1-5-22-201 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 3)Yasuda Veterinary Clinic, 1-5-22 Midorigaoka, Meguro-ku, Tokyo 152-0034, Japan 4)School of Agriculture, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan ABSTRACT. The objectives of this study were to assess if Clinofibrate (CF) treatment improved J. Vet. Med. Sci. lipid metabolism in dogs, and to clarify whether its efficacy is influenced by canine characteristics. 80(6): 945–949, 2018 We collected medical records of 306 dogs and performed epidemiological analyses. Lipid values of all lipoproteins were significantly decreased by CF medication, especially VLDL triglyceride doi: 10.1292/jvms.17-0703 (TG) concentration (mean reduction rate=54.82%). However, 17.65% of dogs showed drug refractoriness in relation to TG level, and Toy Poodles had a lower CF response than other breeds (OR=5.36, 95% CI=2.07–13.90). Therefore, our study suggests that genetic factors may have an Received: 22 December 2017 effect on CF response, so genetic studies on lipid metabolism-related genes might be conducted Accepted: 9 March 2018 to identify variations in CF efficacy. Published online in J-STAGE: KEY WORDS: clinofibrate, descriptive epidemiology, drug response, dyslipidemia, Toy Poodle 26 March 2018 High serum cholesterol (Cho) and triglyceride (TG) concentrations in dogs are caused by various factors such as lack of exercise, high fat diets, obesity, neutralization, age, diseases and breed [6, 21, 24]. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats
Showa Univ. J. Med. Sci. 7(2), 173•`182, December 1995 Original Effects of Clofibrate Derivatives on Hyperlipidemia Induced by a Cholesterol-Free, High-Fructose Diet in Rats Hideyukl KURISHIMA,Sadao NAKAYAMA,Minoru FURUYA and Katsuji OGUCHI Abstract: The effects of the clofibrate derivatives fenofibrate (FF), bezafibrate (BF), and clinofibrate (CF), on hyperlipidemia induced by a cholesterol-free, high-fructose diet (HFD) in rats were investigated. Feeding of HFD for 2 weeks increased the high-density lipoprotein subfraction (HDL1) and decreased the low-density lipoprotein (LDL) fraction. The levels of total cholesterol (TC), free cholesterol, triglyceride (TG), and phospholipid in serum were increased by HFD feeding. Administration of CF inhibited the increase in HDL1 content. All three agents inhibited the decrease in LDL level. Both BF and CF decreased VLDL level. Administration of FF, BF, or CF inhibited the increases of serum lipids, especially that of TC and TG. The inhibitory effects of CF on HFD- induced increases in HDL1, TC, and TG were greater than those of FF and BF. These results demonstrate that FF, BF, and CF improve the intrinsic hyper- lipidemia induced by HFD feeding in rats. Key words: fenofibrate, bezafibrate, clinofibrate, fructose-induced hyperlipide- mia, lipoprotein. Introduction Clofibrate is one of the most effective antihypertriglycedemic agents currently available. However, because of its adverse effects, such as hepatomegaly1, several derivatives, such as clinofibrate (CF) and bezafibrate (BF) have been developed which are more effective and have fewer adverse effects. For example, it has been shown that the hypolipidemic effect of CF is greater than that of clofibrate while its tendency to produce hepatomegaly is less1. -

Joint Assessment Report Was Discussed by the Phvwp at Its Meeting in July 2007 and Finalised in September 2007
ASSESSMENT REPORT on the benefit:risk of fibrates EXECUTIVE SUMMARY 1. BACKGROUND In the light of the established role of statins in the primary and secondary prevention of cardiovascular disease (CVD) and safety concerns arising from the use of fibrates, the CHMP Pharmacovigilance Working Party (PhVWP) agreed to undertake a benefit:risk assessment of this class of medicines. The objective was to establish the current place of fibrates in the treatment of cardiovascular and dyslipidaemic diseases, and in diabetes mellitus; also to provide recommendations regarding amendments of the Summary of Product Characteristics (SPC), as necessary. Fibrates exert their effects mainly by activating the peroxisome proliferator-activated receptor-alpha (PPAR-alpha). Unique in this class, bezafibrate is an agonist for all three PPAR isoforms alpha, gamma, and delta. Fibrates have been shown to reduce plasma triglycerides by 30% to 50% and raise the level of high density lipoprotein cholesterol (HDL- C) by 2% to 20%. Their effect on low density lipoprotein cholesterol (LDL-C) is variable, ranging from no effect to a small decrease of the order of 10%. Today there are four licensed fibrates: bezafibrate, fenofibrate, gemfibrozil and ciprofibrate. Their currently approved indications are quite broad and in many cases still use the old Fredrickson classification for dyslipidaemias. 2. METHODOLOGY In February 2006 a List of Questions was agreed by the PhVWP for the Marketing Authorisation Holders (MAHs) of medicinal products containing one of the four currently licensed fibrates (Annex 1). Other clofibrate-containing medicinal products (e.g. etofibrate, etofyllinclofibrate) were excluded from this class review, since these are available only in a few member states via national marketing authorizations. -

The Effects of Statin and Fibrate Drugs on Cholesterol Metabolism And
The effects of statin and fibrate drugs on cholesterol metabolism and steroid production in two fish species. By Aziz Al-Habsi Thesis submitted to the Faculty of Graduate and Postdoctoral Studies University of Ottawa In partial fulfillment of the requirements for the PhD degree in the Ottawa-Carleton Institute of Biology Thèse soumise à la Faculté des Études Supérieurs et Postdoctorales Université d’Ottawa En vue de la réalisation partielle du doctorat à L’Institut de Biologie Ottawa-Carleton ©Aziz Al-Habsi, Ottawa, Canada, 2014 This thesis is dedicated to my wife and children who have always stood by me and dealt with all my absence from many family occasions with a smile. ii Acknowledgments Completing a PhD is truly a marathon event, and I would not have able to complete this journey without the aid and support of countless people over the past seven years. First and foremost I would like to gratefully and sincerely thank my supervisor Dr. Thomas W. Moon for his guidance, understanding, friendship, and most importantly, his patience during my graduate studies at University of Ottawa. His mentorship was paramount in providing a well-rounded experience consistent my long-term career goals. He encouraged me not only to be a biologist but also be an instructor and independent thinker. I am not sure many graduate students are given the opportunity to develop their own individuality and self- sufficiency by being allowed to work with such independence. For everything you’ve done for me, Dr. Moon, I thank you. I would like to extend my thanks the Department of Biology at University of Ottawa, especially those members of my doctoral committee for their input, valuable discussions and accessibility. -
Bezalip, 200 Mg Film Coated Tablets Bezalip Retard, 400 Mg Sustained Release, Film Coated Tablets
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Bezalip, 200 mg film coated tablets Bezalip Retard, 400 mg sustained release, film coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 200 mg of bezafibrate. Each sustained release, film coated tablets contains 400 mg of bezafibrate. Excipient with known effect: lactose monohydrate (Bezalip Retard) For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Bezalip is a white, round film-coated tablet imprinted with ‘G6’ at reverse. Bezalip Retard is a white, round film-coated tablet engraved D9 on one face. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Bezalip and Bezalip is indicated for: • primary hyperlipidaemia types IIa, IIb, III, IV and V (Fredrickson classification) corresponding to groups I, II and III of the European Atherosclerosis Society guidelines – when diet alone or improvements in lifestyle such as increased exercise or weight reduction do not lead to an adequate response. • secondary hyperlipidaemias, e.g. severe hypertriglyceridemias, when sufficient improvement does not occur after correction of the underlying disorder (e.g. diabetes mellitus). 4.2 Dose and method of administration Adults The standard dosage for Bezalip 200 mg tablets is 1 tablet (200 mg) 3 times daily. In cases of good therapeutic response, especially in hypertriglyceridaemia, the dosage can be reduced to 1 tablet twice daily. For patients with a history of gastric sensitivity, the dosage may be gradually increased to the maintenance level. The standard dosage for Bezalip Retard 400 mg tablets is 1 tablet once daily. Special populations Patient with renal Impairment The dosage in patients with impaired renal function must be adjusted according to serum creatinine levels or creatinine clearance. -

(CPG) for the Management of Dyslipidemia for Cardiovascular
VA/DoD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF DYSLIPIDEMIA FOR CARDIOVASCULAR RISK REDUCTION Department of Veterans Affairs Department of Defense QUALIFYING STATEMENTS The Department of Veterans Affairs and the Department of Defense guidelines are based upon the best information available at the time of publication. They are designed to provide information and assist decision making. They are not intended to define a standard of care and should not be construed as one. Neither should they be interpreted as prescribing an exclusive course of management. This Clinical Practice Guideline is based on a systematic review of both clinical trial and epidemiological evidence. Developed by a panel of multidisciplinary experts, it provides a clear explanation of the logical relationships between various care options and health outcomes while rating both the quality of the evidence and the strength of the recommendation. Variations in practice will inevitably and appropriately occur when clinicians take into account the needs of individual patients, available resources, and limitations unique to an institution or type of practice. Every healthcare professional making use of these guidelines is responsible for evaluating the appropriateness of applying them in the setting of any particular clinical situation. These guidelines are not intended to represent Department of Veterans Affairs nor TRICARE policy. Further, inclusion of recommendations for specific testing and/or therapeutic interventions within these guidelines does not guarantee coverage. Additional information on current TRICARE benefits may be found at www.tricare.mil or by contacting your regional TRICARE Managed Care Support Contractor. Version 4.0 – 2020 VA/DoD Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction Prepared by: The Management of Dyslipidemia for Cardiovascular Risk Reduction Work Group With support from: The Office of Quality and Patient Safety, VA, Washington, DC & Office of Evidence Based Practice, U.S. -

Bempedoic Acid and Inclisiran for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value
Bempedoic Acid and Inclisiran for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value Draft Evidence Report November 12, 2020 Prepared for ©Institute for Clinical and Economic Review, 2020 ICER Staff and Consultants Modeling Team Grace A. Lin, MD, MAS Dhruv S. Kazi, MD, MSc, MS Associate Professor of Medicine and Health Policy Associate Director, Smith Center for Outcomes University of California, San Francisco Research in Cardiology Director, Cardiac Critical Care Unit Jane Jih, MD, MPH Beth Israel Deaconess Medical Center Assistant Professor, Division of General Internal Associate Professor, Harvard Medical School Medicine University of California, San Francisco Foluso Agboola, MBBS, MPH Director, Evidence Synthesis Dr. Kazi was responsible for the development of the ICER cost-effectiveness model, interpretation of results, and drafting of the economic sections of this report; the Rick Chapman, PhD, MS resulting ICER reports do not necessarily represent the Director of Health Economics views of Beth Israel Deaconess Medical Center or ICER Harvard Medical School. Steven D. Pearson, MD, MSc President ICER DATE OF PUBLICATION: November 12, 2020 How to cite this document: Lin GA, Kazi DS, Jih J, Agboola F, Chapman R, Pearson SD. Inclisiran and Bempedoic Acid for Patients with Heterozygous Familial Hypercholesterolemia and for Secondary Prevention of ASCVD: Effectiveness and Value; Draft Evidence Report. Institute for Clinical and Economic Review, November 12, 2020. https://icer-review.org/material/high-cholesterol-update- draft-evidence-report/ Grace Lin served as the lead author for the report and wrote the background, other benefits, and contextual considerations sections of the report, with Jane Jih serving as a co-author. -

(12) United States Patent (10) Patent No.: US 7,795,310 B2 Lee Et Al
US00779531 OB2 (12) United States Patent (10) Patent No.: US 7,795,310 B2 Lee et al. (45) Date of Patent: Sep. 14, 2010 (54) METHODS AND REAGENTS FOR THE WO WO 2005/025673 3, 2005 TREATMENT OF METABOLIC DISORDERS OTHER PUBLICATIONS (75) Inventors: Margaret S. Lee, Middleton, MA (US); Tenenbaum et al., “Peroxisome Proliferator-Activated Receptor Grant R. Zimmermann, Somerville, Ligand Bezafibrate for Prevention of Type 2 Diabetes Mellitus in MA (US); Alyce L. Finelli, Patients With Coronary Artery Disease'. Circulation, 2004, pp. 2197 Framingham, MA (US); Daniel Grau, 22O2.* Shen et al., “Effect of gemfibrozil treatment in sulfonylurea-treated Cambridge, MA (US); Curtis Keith, patients with noninsulin-dependent diabetes mellitus'. The Journal Boston, MA (US); M. James Nichols, of Clinical Endocrinology & Metabolism, vol. 73, pp. 503-510, Boston, MA (US) 1991 (see enclosed abstract).* International Search Report from PCT/US2005/023030, mailed Dec. (73) Assignee: CombinatoRx, Inc., Cambridge, MA 1, 2005. (US) Lin et al., “Effect of Experimental Diabetes on Elimination Kinetics of Diflunisal in Rats.” Drug Metab. Dispos. 17:147-152 (1989). (*) Notice: Subject to any disclaimer, the term of this Abstract only. patent is extended or adjusted under 35 Neogi et al., “Synthesis and Structure-Activity Relationship Studies U.S.C. 154(b) by 0 days. of Cinnamic Acid-Based Novel Thiazolidinedione Antihyperglycemic Agents.” Bioorg. Med. Chem. 11:4059-4067 (21) Appl. No.: 11/171,566 (2003). Vessby et al., “Effects of Bezafibrate on the Serum Lipoprotein Lipid and Apollipoprotein Composition, Lipoprotein Triglyceride Removal (22) Filed: Jun. 30, 2005 Capacity and the Fatty Acid Composition of the Plasma Lipid Esters.” Atherosclerosis 37:257-269 (1980). -
Lipid Management Protocol
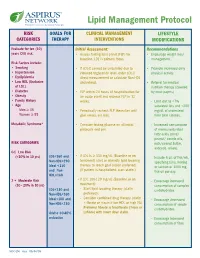
Lipid Management Protocol RISK GOALS FOR CLINICAL MANAGEMENT LIFESTYLE CATEGORIES THERAPY INTERVENTIONS MODIFICATIONS Evaluate for ten (10) Initial Assessment: Recommendations years CVD risk. • Assess fasting lipid panel (FLP) for • Encourage weight loss/ baseline. LDL is primary focus. management. Risk Factors include: • Smoking • If LDL-C cannot be calculated due to • Promote increased daily • Hypertension elevated triglyceride level, order LDL-C physical activity. • Dyslipidemia direct measurement or calculate Non-HDL • Low HDL (Exclusive cholesterol. • Referral for medical of LDL) nutrition therapy (covered • Diabetes • FLP within 24 hours of hospitalization for by most payers) • Obesity an acute event and recheck FLP in 12 • Family History weeks. - Limit diet to <7% • Age saturated fats and <200 Men ≥ 45 • Periodically recheck FLP thereafter until mg/dL of cholesterol Women ≥ 55 goal values are met. from total calories. Metabolic Syndrome* • Consider fasting glucose on all initial - Increased consumption protocols and prn. of monounsaturated fatty acids (olive/ peanut/ canola oils, RISK CATEGORIES nuts/peanut butter, avocado, olives). 0-1 Low Risk (<10% in 10 yrs) LDL<160 and • If LDL is ≥ 130 mg/dL (Baseline or on - Include 6 oz. of fish/wk, Non-HDL<190 treatment) start or intensify lipid lowering specifying tuna, herring Ideal <130 therapy to reach goal (statin preferred). or salmon or 1000 mg and Non- (If patient is hospitalized, start statin.) fish oil per day. HDL<160 2 + Moderate Risk • If LDL 100-129 mg/dL (Baseline or on - Encourage increased (10 - 20% in 10 yrs) treatment): consumption of complex LDL<130 and - Start lipid lowering therapy (statin carbohydrates. Non-HDL<160 preferred).