Evaluation of Universal Extractants for Determination of Selected Micronutrients from Soil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

ETHIOPIA: FOOD Emergency Appeal N° MDRET005 GLIDE N° DR-2008-000043-ETH
ETHIOPIA: FOOD Emergency appeal n° MDRET005 GLIDE n° DR-2008-000043-ETH INSECURITY 19 August 2008 This revised Appeal seeks CHF 8,157,607 (USD 7,920,006 or EUR 5,035,560) in cash, kind or services to support the Ethiopian Red Cross Society (ERCS) to assist 76,075 beneficiaries for 6 months. CHF 300,000 (USD 288,462 or EUR 187,500) was allocated from the Federation’s Disaster Relief Emergency Fund (DREF) to support this operation. Unearmarked funds to replenish DREF are encouraged. Furthermore, this emergency operation has received bilateral contributions from ECHO, through Finnish Red Cross and Austrian Red Cross (EUR 2.7 mill) and the Austrian Development Agency (EUR 200,000). Photo Above: Food distribution in process Based on the situation, this revised appeal responds to a request from ERCS to include the population of neighbouring Damot Gale who live under the same precarious situation as the population of Damot Pulasa and to provide support to 15,215 (7,200 in Damot Gale and 8,015 households in Damot Pulasa) with the total beneficiary of 76,075 (36,000 in Damot Gale and 40,075 in Damot Pulasa). This revised appeal is intended to address emergency food and relief assistance, early recovery activities as well as improving access to safe water and hygiene promotion. This operation is expected to be implemented over 6 months time The first two months’ emergency relief assistance have already been distributed in June and July 2008 in Damot Pulasa while distribution in Damot Gale is expected to start in August 2008. -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

World Bank Document
Sample Procurement Plan (Text in italic font is meant for instruction to staff and should be deleted in the final version of the PP) Public Disclosure Authorized (This is only a sample with the minimum content that is required to be included in the PAD. The detailed procurement plan is still mandatory for disclosure on the Bank’s website in accordance with the guidelines. The initial procurement plan will cover the first 18 months of the project and then updated annually or earlier as necessary). I. General 1. Bank’s approval Date of the procurement Plan [Original: December 2007]: Revision 15 of Updated Procurement Plan, June 2010] 2. Date of General Procurement Notice: Dec 24, 2006 3. Period covered by this procurement plan: The procurement period of project covered from year June 2010 to December 2012 Public Disclosure Authorized II. Goods and Works and non-consulting services. 1. Prior Review Threshold: Procurement Decisions subject to Prior Review by the Bank as stated in Appendix 1 to the Guidelines for Procurement: [Thresholds for applicable procurement methods (not limited to the list below) will be determined by the Procurement Specialist /Procurement Accredited Staff based on the assessment of the implementing agency’s capacity.] Procurement Method Prior Review Threshold Comments US$ Public Disclosure Authorized 1. ICB and LIB (Goods) Above US$ 500,000 All 2. NCB (Goods) Above US$ 100,000 First contract 3. ICB (Works) Above US$ 15 million All 4. NCB (Works) Above US$ 5 million All 5. (Non-Consultant Services) Below US$ 100,000 First contract [Add other methods if necessary] 2. -

Study on Ruminal and Reticular Foreign Bodies in Cattle Slaugthered at Boditii Municipal Abattoir, Wolaita Zone, Southern Ethiopia
Middle-East Journal of Scientific Research 28 (2): 80-89, 2020 ISSN 1990-9233 © IDOSI Publications, 2020 DOI: 10.5829/idosi.mejsr.2020.80.89 Study on Ruminal and Reticular Foreign Bodies in Cattle Slaugthered at Boditii Municipal Abattoir, Wolaita Zone, Southern Ethiopia 12Temesgen Zekarias, Tadewos Tantasa and 3Amanuel Ashebo 1Animal Health Researcher, Ethiopian Institute of Agricultural Research, Addis Ababa, Ethiopia 2Animal Health Expert, Livestock and Fisheries Development Office, Humbo, Wolayta Zone, Ethiopia 3Animal Health Expert, livestock and Fisheries Development Office Hadero Tunto Zuria Woreda, Kembeta Tembaro Zone SNNPR, Tunto, Ethiopia Abstract: A cross-sectional study was conducted from November, 2017 to April, 2018 at Boditi Municipal Abattoir, Wolaita zone, Southern Ethiopia. With the objectives of to assess the prevalence and type of ruminal and reticular foreign bodies in cattle slaughtered at Boditi Municipal Abattoir. The study animals were selected by using systematic random sampling method from the total slaughtered animals. From the total of 514 male animals that were examined, 20.8% (n=107) were had foreign bodies at slaughter. When the prevalence was compared between breed, among different age groups, among different body condition score and animal originated from different areas, higher prevalence of foreign bodies 39.6%, 27.2%, 40.1%, 26.1% were observed in cross breed, old animals, animals having poor body condition and animals originate from Sodo, respectively. These factors are considered as potential risk factors and found highly significantly associated (P<0.05) with the occurrence of foreign bodies. The rumen harbored mostly non-metallic materials while reticulum was the major site for the retention of metallic objects. -

An Assessment of Rural Households Livelihood Assets of Wolaita Zone, Ethiopia
Research on Humanities and Social Sciences www.iiste.org ISSN 2224-5766 (Paper) ISSN 2225-0484 (Online) Vol.10, No.5, 2020 An Assessment of Rural Households Livelihood Assets of Wolaita Zone, Ethiopia Deneke Dana Dabara (PhD) Economic Geography, Wolaita Sodo University, Ethiopia Abstract The aim of this study was to assess existing rural household’s livelihood assets in Wolaita zone, Ethiopia. The demographic and socio-economic data were collected from 300 randomly selected rural households. The basic data for the study were obtained from both primary and secondary sources. The collected data were analysed by using both qualitative and quantitative methods. The study revealed land is one of the basic economic assets of and livelihood sources for agrarian rural people but the size of the plots is so small in the study area. The findings concerning the livelihood assets that had been developed were presented in line with the three livelihood assets, namely human, natural, and financial capital, all of which were shown to have negative results. The above- mentioned livelihood assets in the study area were weak. From the human assets section, 41percent of the study population had not achieved a school educational qualification. About half percent of respondents said their soil was not fertile from the physical assets perspective. A large number of respondents did not practise saving on account of their low daily-income status. Thus concerning body including government should strongly work on the discussed rural assets built up to minimize the rural household’s livelihood problems in study area. Keywords: Wolaita, rural, assets, human, natural, financial, households, livelihood DOI: 10.7176/RHSS/10-5-01 Publication date: March 31 st 2020 1.INTRODUCTION Of all the less-developed regions of the world, Sub-Saharan Africa is the most severely affected region in terms of livelihood problems. -

Ethiopia: SNNP Region Administrative Map (As of 15 Aug 2017)
Ethiopia: SNNP region administrative map (as of 15 Aug 2017) ! ! ! ! ! ! ! ! ! Suten ! ! ! ! ! ! Inge Sodo ! ! !Bui ! ! WelikiteKebena Abeshege ! Kokir Gedbano ! ! Kela ! ! Muhur Na Ak!lil ! Gubire ! ! ! Cheha Agena ! Imdibir! ! Ezha Me!skan ! ! Inseno ! Gonichire ! ! ! Kibet Qewaqoto! Koshe ! ! ! ! ! ! ! Enemorina Eaner Alicho Woriro ! Gumer Mareko ! Selti ! ! Areket Alkeso town ! ! ! ! ! ! Geta Kose Tora ! Fofa ! Werabe ! ! ! Dinkela ! ! Sayilem! ! ! ! ! Yadota Geja Endiguagn Yem SP Woreda ! Dalocha ! Misrak Azenet Berbere ! ! ! ! Misha !LERA Dalocha Masha ! Wilb!areg Gibe ! ! Mierab Azenet Berbere ! ! Lanfero ! Homec!ho ! ! Fonqo town ! Mito ! GAMBELA Gesha (Deka) Kondo GECHA TOWN ! Analemmo ! ! !Deka ! Doesha !Belesa town ! Alem Gebeya Anderacha Getawa Gembora ! ! Limu ! ! Bonosha Sankura ! ! ! Lisana town Jajira Shashogo Gimbichu! ! Hufa ! ! ! Diri Soro ! Gojeb Bita (Big) Gimbo Doya Gena Jacho A!nigach!a ! Alaba SP Woreda ! ! ! Daniboya Wishiwishi Dune Kulito ! Kaka Idget ! Bita Genet ! OROMIA Kelata Mudula Hobichaka ! ! Bonga ! ! ! ! ! Yeki ! Menjiwo ! Chena Tembaro Ke!diada Gambela TEPI TOWN Hadero !TubitoKacha Bira ! ! ! !Adilo Chda Idge T!unito ! Legend WACHA ! ! Terche Misrak Badawacho ! Gena Bosa Chiri BOMIBE 01 ! ! ! ! !Karewo ! Mierab Badawacho ! Ameya P ! Tocha Tocha Edget Boloso Bombe Sheka Tulo ! Regional capital ! Waka ! Semen Bench Alem Gena ! ! ! ! Mehal Sheko Mareka Boloso SoreDamot Pulasa Hawassa Zuria PWondo-Ge! net Gesa ! ! Shanito Hawasa Town ! ! ! ! Shama Chuko Shay Bench ! Bitena Town Mizan Aman ! ! Tula ! Damot -
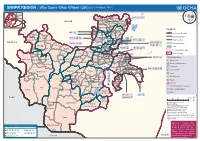
SNNPR REGION : Who Does What Where (3W) (As of 01 March 2012)
(as of 01 March 2012) SNNPR REGION : Who Does What Where (3W) Tigray Afar Amhara Sodo! ECS: a cç Benshangul Gumuz KebenaKokir Gedbano Dire Dawa Abeshege Addis Ababa Hareri Gambela Oromia Oromia Muhur Na Aklil Somali Cheha SNNPR Gurage Ezha Meskan Alicho Woriro Enemorina Eaner Gumer Selti Mareko Yem Geta Selti Legend Sayilem ! IRC: ç Endiguagn Dalocha Yem SP Wor!eda International Boundary Masha Gibe Misha Wilbareg Lanfero Regional Boundary Sheka Gesha (Deka) ECS: ah ç HadiyaAnalemmo ! Anderacha Getawa Gembora ! Sankura LVIA: a 4 l Zonal Boundary ! Limu Gambella Shash! ogo LVIA: a 4 l Plan Int.: : h Soro Woreda Boundary Gimbo Anigacha Alaba SP Woreda SC UK: h Bita (Big) ! Lake Dune Daniboy! a Alaba KT Plan Int.: h IMC: î h ç Yeki Chena Menjiwo Tembaro Keffa Kacha BiraKediada Gambela No Intervention/No Data Gena Bosa Misrak Badawacho Tocha Boloso Bom! be Other Region Sheka Tulo Wondo-Genet Semen Bench Boloso sore Awassa Zuria Mareka A! wasa Town Dawro Damot Gale Plan Int.: d Clusters/Sectors Ela (Konta) SP Woreda Kindo Koysha Diguna Fango ! Malga Gurafereda Debub BenchShay Bench Cheta Boricha Agriculture Decha Esira Damot Sore a Konta Loma Bosa Sodo ZuriaDamot Weydie Shebe DinoGorche Wolayita Dale : Disaster Management Menit Goldiye Kindo Dida Ofa Humbo Wonosho Arbe Gonna d Education ! Loka-Abaya ! Bursa Sidama ACF: aîlf Chuko 4 Environment Melekoza Kucha Boreda Bensa Menit Shasha ! Hulla Denibu Gofa Dara Bona Zu! ria ç Chire î Food Dila Zuria Bero BasketoGeze Gofa h Aroresa l Food Security Zala Mirab Abaya Wenago Basketo SP Woreda DaramaloDita -

Guide 2000 English.Pub
1 MAY 2008 AWASSA 2 Table of Contents Page N0 • Introduction 1 Part I Location and Administrative Division—- - - - - - - - - - - - - - 2 • Topography —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2 • Climate —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - • Soil Resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3 • Land use and land cover —- - - - - - - - - - - - - - - - - - - - - - - - - 3 • Water bodies —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - • Forest Resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5 • Wild life resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5 • Tourist Attraction and potential —- - - - - - - - - - - - - - - - - - - 6 • Population —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7 • Ethnic Composition —- - - - - - - - - - - - - - - - - - - - - - - - - 8 • Urbanization —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8 • Agriculture —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8 • Livestock resource —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Fisher —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Hides and skins production and market —- - - - - - - - - - - - - - 9 • Apiculture —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Industry —- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 9 • Education—- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -

Socioeconomic Determinants of Food Insecurity Among Rural Households in Wolaita Zone, Southern Ethiopia
Economics 2019; 8(2): 55-61 http://www.sciencepublishinggroup.com/j/eco doi: 10.11648/j.eco.20190802.13 ISSN: 2376-659X (Print); ISSN: 2376-6603 (Online) Socioeconomic Determinants of Food Insecurity Among Rural Households in Wolaita Zone, Southern Ethiopia Ermias Ganamo Gazuma *, Ayana Anteneh Astatike Department of Economics, Wolaita Sodo University, Wolaita Sodo, Ethiopia Email address: *Corresponding author To cite this article: Ermias Ganamo Gazuma, Ayana Anteneh Astatike. Socioeconomic Determinants of Food Insecurity Among Rural Households in Wolaita Zone, Southern Ethiopia. Economics . Vol. 8, No. 2, 2019, pp. 55-61. doi: 10.11648/j.eco.20190802.13 Received : April 30, 2019; Accepted : June 2, 2019; Published : June 17, 2019 Abstract: The major aim of this study was to assess the socioeconomic determinants of food insecurity among rural households in Wolaita Zone, Southern Ethiopia. Data for the study was obtained from 168 randomly selected rural households by using an interview schedule. To select sample respondents, the multi-stage sampling technique was used. The Foster-Greer- Thorbecke (FGT) index and binary logistic regression model were used for data analysis. The finding from FGT index showed that about 66.1 percent of households in the study area were found food insecure and the remaining 33.9 percent were found food secure. The depth and severity of food insecurity were found 15 percent and 4.6 percent, respectively. The logistic regression model result revealed that factors such as land size, income from productive safety net program, credit access, farm income, and non-farm income are the statistically significant factors influencing the households’ food insecurity status. -

Refugee Update up to 15,000 People from the Lou Nuer Community Have Crossed Into Ethiopia’S Western Gambella Region Since Mid- February 2012
Refugee Update Up to 15,000 people from the Lou Nuer community have crossed into Ethiopia’s western Gambella Region since mid- February 2012. The new arrivals – most of whom are women, children and elderly – have settled near the border town of Matar and are living in makeshift shelters. Local communities have been sharing existing food and water stocks and other public facilities with the new arrivals, but the influx has stretched water and sanitation facilities beyond their capacity. UNHCR is working with the government refugee agency, ARRA, to establish a transit centre near Matar and to expedite the screening, registration and relocation of individuals verified as refugees to Fugnido camp. As of 13 March, about 1,300 people had been transferred to Fugnido. Fugnido camp was opened in 1993 in response to previous influxes of refugees from Sudan and is home to an existing population of some 23,000 refugees, most of whom would now be Southern Sudanese. UNHCR has started distribution of non-food items (NFIs), including tents, plastic sheets, blankets, kitchen sets and jerry cans to the new arrivals in Fugnido from its stockpile in Gambella, while ARRA and WFP are providing food assistance. WFP is also working with Government counterparts to extend food assistance in Gambella Region to cover the already-vulnerable host community. UNHCR and ARRA are also working to prepare for possible additional influxes of refugees in the future. For more information, contact: [email protected] or [email protected] Price Update Ethiopia’s year-on-year inflation rate rose to 36.3 per cent in February, up from 32 per cent the previous month. -

Value Chain Analysis of Beef Cattle Production in Wolaita Zone: the Case of Damot Gale District, Wolaita Sodo, Boditi and Areka Towns, Southern Ethiopia
Reasearch Article Volume 7 Issue 1 - November 2017 Int J Environ Sci Nat Res DIO : 10.19080/IJESNR.2017.07.555704 Copyright © All rights are reserved by Zekarias Bassa Value Chain Analysis of Beef Cattle Production in Wolaita Zone: The Case of Damot Gale District, Wolaita Sodo, Boditi and Areka Towns, Southern Ethiopia Zekarias Bassa*, Alemayehu Bashe and Tesfaye Alemu Zekarias Bassa*, Alemayehu Bashe and Tesfaye Alemu Areka Agricultural Research center, Ethiopia, Africa Submission: October 30, 2017; Published: November 30, 2017 *Corresponding author: Zekarias Bassa, Areka Agricultural Research Center, Africa, Tel: ; Fax: 251465520502; Email: Abstract Beef cattle production and value addition practices are known as major income earning activities and economic stay of the farmers, brokers, is not comparable to the economic value the sector own. This study, therefore, was initiated to identify value chain actors and their function in the chain,traders, investigate hotels and factors restaurants affecting and value also provideadded in huge beef financial production support and economic town for administrativerole beef value inchain Wolaita for districts. area, yet Both attention primary given and to secondarythe sector data were used for this study. Primary data were collected from 102 sampled farmers, 21 hotels and restaurants, 3 town administrative (Wolaiyta Sodo, Areka, Boditi) and 15 experts from different strata. Before the household survey, key informant interview and focus group discussions were conducted using 20 small scale farmers, 5 hotels and restaurants, 1 town administrative and 1 expert. Keywords: Beef Cattle; Value Chain; Value Added; Income; Market; Institution Introduction administrative as revenue source that calculated to be more than Descriptive Statistics and Econometric data analysis one million Ethiopian Birr in Areka annually.