Innovations November 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glaadawards March 16, 2013 New York New York Marriott Marquis
#glaadawards MARCH 16, 2013 NEW YORK NEW YORK MARRIOTT MARQUIS APRIL 20, 2013 LOS AnGELES JW MARRIOTT LOS AnGELES MAY 11, 2013 SAN FRANCISCO HILTON SAN FRANCISCO - UnION SQUARE CONNECT WITH US CORPORATE PARTNERS PRESIDENT’S LETTer NOMINEE SELECTION PROCESS speCIAL HONOrees NOMINees SUPPORT FROM THE PRESIDENT Welcome to the 24th Annual GLAAD Media Awards. Thank you for joining us to celebrate fair, accurate and inclusive representations of the lesbian, gay, bisexual and transgender (LGBT) community in the media. Tonight, as we recognize outstanding achievements and bold visions, we also take pause to remember the impact of our most powerful tool: our voice. The past year in news, entertainment and online media reminds us that our stories are what continue to drive equality forward. When four states brought marriage equality to the election FROM THE PRESIDENT ballot last year, GLAAD stepped forward to help couples across the nation to share messages of love and commitment that lit the way for landmark victories in Maine, Maryland, Minnesota and Washington. Now, the U.S. Supreme Court will weigh in on whether same- sex couples should receive the same federal protections as straight married couples, and GLAAD is leading the media narrative and reshaping the way Americans view marriage equality. Because of GLAAD’s work, the Boy Scouts of America is closer than ever before to ending its discriminatory ban on gay scouts and leaders. GLAAD is empowering people like Jennifer Tyrrell – an Ohio mom who was ousted as leader of her son’s Cub Scouts pack – to share their stories with top-tier national news outlets, helping Americans understand the harm this ban inflicts on gay youth and families. -
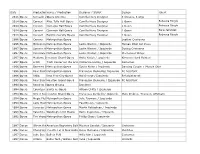
Date Production Name Venue / Production Designer / Stylist Design
Date ProductionVenue Name / Production Designer / Stylist Design Talent 2015 Opera Komachi atOpera Sekidrera America Camilla Huey Designer 3 dresses, 3 wigs 2014 Opera Concert Alice Tully Hall Opera Camilla Huey Designer 1 Gown Rebecca Ringle 2014 Opera Concert Carnegie Hall Opera Camilla Huey Designer 1 Gown Rebecca Ringle 2014 Opera Concert Carnegie Hall Opera Camilla Huey Designer 1 Gown Sara Jakubiak 2013 Opera Concert Bard University Opera Camilla Huey Designer 1 Gown Rebecca Ringle 1996 Opera Carmen Metropolitan Opera Leather Costumes 1996 Opera Midsummer'sMetropolitan Night's Dream Opera Leslie Weston / Izquierdo Human Pillar Set Piece 1997 Opera Samson & MetropolitanDelilah Opera Leslie Weston / Izquierdo Dyeing Costumes 1997 Opera Cerentola Metropolitan Opera Leslie Weston / Izquierdo Mechanical Wings 1997 Opera Madame ButterflyHouston Grand Opera Anita Yavich / Izquierdo Kimonos Hand Painted 1997 Opera Lillith Tisch Center for the Arts OperaCatherine Heraty / Izquierdo Costumes 1996 Opera Bartered BrideMetropolitan Opera Sylvia Nolan / Izquierdo Dancing Couple + Muscle Shirt 1996 Opera Four SaintsMetropolitan In Three Acts Opera Francesco Clemente/ Izquierdo FC Asssitant 1996 Opera Atilla New York City Opera Hal George / Izquierdo Refurbishment 1995 Opera Four SaintsHouston In Three Grand Acts Opera Francesco Clemente / Izquierdo FC Assistant 1994 Opera Requiem VariationsOpera Omaha Izquierdo 1994 Opera Countess MaritzaSanta Fe Opera Allison Chitty / Izquierdo 1994 Opera Street SceneHouston Grand Opera Francesca Zambello/ Izquierdo -
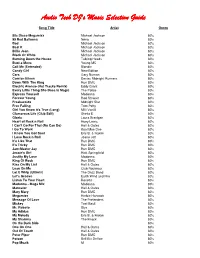
MP3 Database.Wdb
Audio Tech DJ's Music Selection Guide Song Title Artist Genre 80s Disco Megamixx Michael Jackson 80's 99 Red Balloons Nena 80's Bad Michael Jackson 80's Beat It Michael Jackson 80's Billie Jean Michael Jackson 80's Black Or White Michael Jackson 80's Burning Down the House Talking Heads 80's Bust a Move Young MC 80's Call Me (Extended) Blondie 80's Candy Girl New Edition 80's Cars Gary Numan 80's Com'on Eileen Dexies Midnight Runners 80's Down With The King Run DMC 80's Electric Avenue (Hot Tracks Remix) Eddy Grant 80's Every Little Thing She Does Is Magic The Police 80's Express Yourself Madonna 80's Forever Young Rod Stewart 80's Freakazoids Midnight Star 80's Free Falling Tom Petty 80's Girl You Know it's True (Long) Milli Vanilli 80's Glamorous Life (Club Edit) Sheila E 80's Gloria Laura Branigan 80's Heart of Rock n Roll Huey Lewis 80's I Can't Go For That (No Can Do) Hall & Oates 80's I Go To Work Kool Moe Dee 80's I Know You Got Soul Eric B. & Rakim 80's I Love Rock n Roll Joane Jett 80's It's Like That Run DMC 80's It's Tricky Run DMC 80's Jam-Master Jay Run DMC 80's Jessie's Girl Rick Springfield 80's Justify My Love Madonna 80's King Of Rock Run DMC 80's Kiss On My List Hall & Oates 80's Lean On Me Club Nouveau 80's Let It Whip (Ultimix) The Dazz Band 80's Let's Groove Earth Wind and Fire 80's Listen To Your Heart Roxette 80's Madonna - Mega Mix Madonna 80's Maneater Hall & Oates 80's Mary Mary Run DMC 80's Megamixx Herbie Hancock 80's Message Of Love The Pretenders 80's Mickey Toni Basil 80's Mr. -

Working for the IAEA
Working for the IAEA A Guide for US Citizens 2018 Edition Working for the IAEA A Guide for US Citizens 2018 Edition From the Editors This Guidebook is intended to provide practical information for United States citizens embarking on or considering an assignment at the International Atomic Energy Agency (IAEA) in Vienna, Austria. Since its first appearance in 1989, the Guidebook has been revised frequently to reflect changes occurring at the IAEA, within the United States Support Program to IAEA Safeguards (USSP), and in Vienna. The 2018 Edition reflects these changes at the time of publication. Nevertheless, IAEA salaries, allowances, and other benefits change, as do telephone numbers, addresses, and websites. Currency exchange rates, prices, and store hours in Vienna inevitably fluctuate. We regret any inconvenience this may cause our readers. The 2018 Edition of the Guidebook was prepared by the International Safeguards Project Office (ISPO) under the auspices of the USSP and was published by Brookhaven National Laboratory (BNL). Jeanne Anderer, Ben Dabbs Editors November 2018 Working for the IAEA: A Guide for US Citizens 2018 Edition Prepared by the International Safeguards Project Office (ISPO) under the auspices of the United States Support Program to IAEA Safeguards (USSP) International Safeguards Project Office (ISPO) Brookhaven National Laboratory 30 Bell Avenue, Building 490C Upton, New York 11973‑5000, USA Telephone: (631) 344‑5902 Fax: (631) 344‑5266 Web: bnl.gov/ispo facebook.com/ISPObnl youtube.com/IAEAvideo Printed by Brookhaven -

Cure Research Advancing on Multiple Fronts Dame Elizabeth
SpRInG 2011 INNThe newsletter of amfAR,OV The FoundationAT for AIDS ResearchIONS Dame Elizabeth Taylor, 1932 –2011 Founding International Chairman, amfAR Dame Elizabeth Taylor was one of the most inspirational fi gures in the fi ght against HIV/AIDS. In the early years of the epidemic, she brought AIDS out of the shadows and used her celebrity to shame and cajole politicians into action. An ardent and forceful advocate, Dame Elizabeth testifi ed before Congress on the need for substantial investments in AIDS treatment, care, and research, and she raised millions for amfAR through her participation in benefi t events in the U.S. and abroad. Unfortunately, ill health prevented Dame Elizabeth from attending the amfAR New York Gala in February, when she was honored in absentia with amfAR’s Award of Courage. In his tribute remarks, Sir Elton John said, “Elizabeth Taylor was a force of nature. She compelled people to listen, made them respond, urged them to act. Elizabeth Taylor has inspired every one of us and many millions of others around the world. She has shown us the meaning of courage and moral responsibility. She’ll forever be our guiding star.” n (For more on Elizabeth Taylor’s extraordinary contributions to the fi ght against HIV/AIDS, see insert.) Cure Research Advancing on Multiple Fronts Photo: Monash University Scientifi c interest in the search for a cure for December 2010 publication in the journal HIV/AIDS has been building over the past Blood of a peer-reviewed paper on the “Berlin few years, a groundswell that is generating patient”—the HIV-positive man whose cure a critical mass of activity among researchers via a stem-cell transplant inspired new hope and scientifi c organizations, including amfAR. -

Victoria's Secret Fashion Show Media Kit 2013
ADRIANA LIMA ALESSANDRA AMBROSIO Adriana Lima, originally from SALVADOR, BAHIA, BRAZIL, Born in ERECHIM, BRAZIL, to parents of Italian and Polish moved to New York City to pursue a modeling career descent, Alessandra Ambrosio nurtured her desire to after winning a 1996 modeling contest in her native become a model from a young age. After enrolling herself country. in modeling classes, her career launched at the renowned “ELITE MODEL LOOK” in Brazil. In 1999, Adriana began working with Victoria’s Secret and has been an officialVICTORIA’S SECRET ANGEL Alessandra has been a Victoria’s Secret Angel for more SINCE 2000. She has opened the annual Victoria’s than 10 years and continues to be a global spokesmodel Secret Fashion Show on multiple occasions and donned for all things Victoria’s Secret. As the first ambassador for the coveted million-dollar Fantasy Bra twice. the Victoria’s Secret Pink line, she helped launch the brand during a nationwide tour in 1999. Though Adriana is most widely associated with Victoria’s Secret, she has also worked for brands such as Christian She has also walked in shows for some of the biggest Dior, Louis Vuitton, Giorgio Armani, Fendi, Givenchy, Vera designers in fashion, such as Dolce & Gabbana, Marc Wang and Valentino. Adriana has appeared on covers Jacobs, Prada and Louis Vuitton. Alessandra has been and in features of several INTERNATIONAL EDITIONS of featured in 70 INTERNATIONAL MAGAZINES, including Vogue, Harper’s Bazaar, Elle, Marie Claire and W Magazine. Harper’s Bazaar, Cosmopolitan, Elle, Rolling Stone and several editions of international Vogue. -

Sharon Stone, Catherine Deneuve, Anastacia Y Naomi Campbell
10754369 05/20/2006 10:34 p.m. Page 3 ESPECTÁCULOS | DOMINGO 21 DE MAYO DE 2006 | EL SIGLO DE DURANGO | 3B Naomi Campbell imprime su rostro en un sello postal para aportar algo al suceso. ORGANIZADOR | ELTON JOHN APADRINA EL SUCESO Famosos bailarán contra el Sida Sharon Stone, La modelo Naomi Camp- bell -anunciaron los organiza- Granito Catherine Deneuve, dores- protagonizará una se- de arena Anastacia y Naomi rie limitada de sellos que crearán los correos austría- Cada celebridad ayudará con Campbell participan cos y cuyos fondos irán a la lo que mejor sabe hacer a causa benéfica. esta campaña contra el Sida. en evento benéfico La cantante estadouni- dense Anastacia dijo que ne- cesitan “la voz y el apoyo de ■ Sharon Stone será la EFE todos”, y lanzó un mensaje de portavoz del evento a “conscientización” para que nivel mundial. VIENA, AUSTRIA.- Las actrices las personas se impliquen en ■ Naomi Campbell Sharon Stone y Catherine De- más proyectos solidarios. protagonizará una serie neuve, la cantante Anastacia La reconocida actriz fran- limitada de sellos que y la modelo Naomi Campbell cesa Catherine Deneuve será crearán los correos son algunas de las invitadas la encargada de entregar en la austríacos y modeló en Viena a la fiesta benéfica gala el premio “Cristal de la anoche para la marca “Baile por la vida”, apadrina- esperanza” a Medicos Sin Diesel, en el evento da por el cantante Elton John, Fronteras, dotado con 100 mil inicial. con el fin de recaudar fondos euros donados por la marca de ■ Anastacia también para la lucha contra el Sida. -

SACMAT 2015 Venue Information
SACMAT 2015 Venue Information 01-03 June 2015 Vienna, Austria sacmat.org Content Venue Overview ....................................................................................................... 1 Conference Venue .................................................................................................... 2 Directions ............................................................................................................... 3 Public Transport ....................................................................................................... 9 Welcome to Vienna! ............................................................................................... 10 Useful Information .............................................................................................. 10 Public Transport Tickets ...................................................................................... 11 About Vienna ....................................................................................................... 12 Cultural Program taking place from June 1 – 3, 2015 ........................................... 16 Cafe Concerts, Heurigen & Dinner Shows .......................................................... 17 Exhibitions .......................................................................................................... 19 Sightseeing ......................................................................................................... 20 Conference Office / Contact .................................................................................. -
Parody As a Borrowing Practice in American Music, 1965–2015
Parody as a Borrowing Practice in American Music, 1965–2015 A dissertation submitted to the Graduate School of the University of Cincinnati in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Division of Composition, Musicology, and Theory of the College-Conservatory of Music by John P. Thomerson BM, State University of New York at Fredonia, 2008 MM, University of Louisville, 2010 Committee Chair: bruce d. mcclung, PhD ABSTRACT Parody is the most commonly used structural borrowing technique in contemporary American vernacular music. This study investigates parody as a borrowing practice, as a type of humor, as an expression of ethnic identity, and as a response to intellectual trends during the final portion of the twentieth century. This interdisciplinary study blends musicology with humor studies, ethnic studies, and intellectual history, touching on issues ranging from reception history to musical meaning and cultural memory. As a structural borrowing technique, parody often creates incongruity—whether lyrical, stylistic, thematic, evocative, aesthetic, or functional—within a recognized musical style. Parodists combine these musical incongruities with other comic techniques and social conventions to create humor. Parodists also rely on pre-existing music to create, reinforce, and police ethnic boundaries, which function within a racialized discourse through which parodists often negotiate ethnic identities along a white-black binary. Despite parody’s ubiquity in vernacular music and notwithstanding the genre’s resonance with several key themes from the age of fracture, cultivated musicians have generally parody. The genre’s structural borrowing technique limited the identities musicians could perform through parodic borrowings. This study suggests several areas of musicological inquiry that could be enriched through engagement with parody, a genre that offers a vast and largely unexplored repertoire indicating how musical, racial, and cultural ideas can circulate in popular discourse. -
2019 ANNUAL REPORT Amfar, the Foundation for AIDS Research Cover Photo: Human Cells
2019 ANNUAL REPORT amfAR, The Foundation for AIDS Research Cover photo: Human cells. 3D illustration © Ustyna Shevhcuk | Dreamstime.com Contents amfAR in 2019 01 Grants, Fellowships, and Awards 09 Research Grants, Fellowships, and Awards TREAT Asia Grants and Awards Public Policy Awards Financial Highlights 15 Leadership and Advisory Committees 17 Board of Trustees Scientific Advisory Committee Program Advisory Council Management Group Our Mission: amfAR, The Foundation for AIDS Research, is dedicated to ending the global AIDS epidemic through innovative research. HIGHLIGHTS RESEARCH edge technology to address the main barrier to an HIV cure: the persistent reservoirs of virus not cleared by antiretroviral therapy (ART). Totaling $800,000, this new round of Investment grants launched the critical third phase of a research project begun Countdown to a Cure for AIDS in 2017. In 2015, amfAR launched its Countdown to a Cure for AIDS initiative, which is aimed at developing the scientific basis for a cure. To date, In the first two phases of her study, Dr. Hui Zhang of Johns amfAR has awarded 79 Countdown grants totaling more than $48 Hopkins University used mass spectrometry to scan the surface of million to support research conducted by 277 scientists working human cells for proteins—biomarkers—that discriminate between at 95 institutions in 15 countries. Structured to provide sustained latently infected and uninfected cells. After scanning a variety support for a wide range of studies that advance both emerging and of cell lines, she identified 17 potential targets. In phase three, established ideas, the strategy comprises the following components: Dr. Zhang is teaming up with HIV scientist Dr. -
Downloadable Version of the Complete Summer 2009 Issue
The Newsletter of amfAR, The Foundation for AIDS Research • Summer 2009 Vol. 7, No. 1 INNOVATIONS March 2009 ISSN:1546-3745 amfAR Issues Call to Action at Capitol Hill Summit Nancy Pelosi, “Magic” Johnson, Jeffrey Crowley, Dr. Anthony Fauci Among Speakers National leaders and HIV/AIDS experts called on the federal gov- ernment to take swift action to fulfill President Obama’s pledge to strengthen the domestic AIDS response at a Washington, D.C., confer- ence on May 13, co-sponsored by amfAR and Research!America. Participants emphasized the urgency of implementing an evidence-based national AIDS strategy aimed at lowering the incidence of HIV infection, increasing access to care, and reducing the profound racial disparities of the epidemic. The landmark summit was the first major conference to address the future of the fight against HIV/AIDS since the new administration took office. “Today’s conference gives us hope that by increasing our investments in research and marshalling the skills of these experts, Moderator Ted Koppel, senior we can make tremendous strides in prevention, treatment, and care news analyst for NPR, and of HIV/AIDS,” said conference chair Dr. Susan Blumenthal, amfAR’s senior policy keynote speaker Earvin “Magic” Johnson discussed HIV and and medical adviser. stigma, particularly in the U.S. Speaker of the House Nancy Pelosi (D-CA), who received amfAR’s Award African-American community. (Photo: Paul Morigi/WireImage) of Courage along with Earvin “Magic” Johnson, Senator Edward Kennedy (D-MA), and former U.S. Surgeon General Dr. C. Everett Koop, discussed the importance of CONTINUED ON PAGE 3 From Cameroon to Kazakhstan, MSM Initiative Extends Its Reach In Memoriam New Round of Awards Targets Eastern Europe and Central Asia It is well established that HIV epidemics world, amfAR launched its MSM worldwide are concentrated among Initiative in 2007. -

Charlene of Monaco Water Biker
+SA's HEALTHIEST CITY May 2018 TORCH Charlene of Monaco Water Biker. Paddle Boarder. FAT Olympian.Mother. FAST HOW TO EASY WAYS TO SLAY YOUR TRIM DOWN INNER & TONE UP! QUITTER Sculpt a Tight Tush In 15 Minutes SPECIAL a Day! • Thali’s Best Curries SPECIAL REPORT • Lisa Clark’s Has Social Fave Tomato Media Made Dishes Us More • 30-Minute Lonely? Meals R50.50 (VAT INCL) NAMIBIA N$53.00 • All the 05093 Bliss Balls 9 772075 406001 May 2018 FOOD HUB 40 Taste Of Thali Take a holiday to India via your plate – no passport required 40 44 You Say... Tomato Spice up One super sauce, so many moreish meals your life! 50 All In A Day Lunch, dinner and dessert are sorted with these quick, 30-minute meals 52 Bite-Sized Bliss Craving something sweet? Keep these BEST BODY 86 The Mom Divide nifty nuggets around 58 The Food Lovers’ Workout Want to keep your bestie and your baby happy? Read this 55 10 Kitchen Gadgets You Not even brownies can slow the results Can’t Live Without of this kilojoule-crushing workout 88 Let It Out Luxe essentials you didn’t know you 62 One-Piece Workout: Kettlebell Your guide to healthy venting absolutely needed One move. One tool. A world of gains 90 Drive It Home 64 15-Minute Workout The car you want with the budget you’ve got Make your bod’s biggest muscle work ON OUR a little harder with this quickie STYLE & BEAUTY COVER 65 Back To Basics Increase your balance and stability with this move 93 Break A Nail (Rut) 126 Ditch your fail-safe shades and enter 66 Making A Racquet manicure utopia Trust squash pro Milnay Louw – this is the sport you need to try 96 Just Desserts 58 32 Subtly sweet fragrances to have you smelling 68 Water Hazard like a treat 74 We take a closer look at how water shortages are affecting your health 100 Feed Your Face Nourish hungry skin with these nutrients 71 Your Body On..