Diagnosis and Management of Hepatitis C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Interferon-Based and Interferon-Free New Treatment Options
Interferon-based and interferon-free new treatment options White Nights of Hepatology St. Petersburg, 7. June 2013 Christoph Sarrazin Klinikum der J. W. Goethe-Universität Medizinische Klinik I Frankfurt am Main Mode of action of Interferons natural immunomodulatory effects IFN-stimulated gene activation Antiviral Apoptotic Immunomodulatory activity activity activity B-cell proliferation Reduced transcription Elevates apoptosis by CTL proliferation Reduced translation multiple mechanisms MHC upregulation Reduced RNA stability Augments NK activity Host-mediated effects are important for DAA combination therapy • Potency and additive effects • Prevention of resistance and viral breakthrough Different types of Interferons Type I Interferons Type III Interferons Broad receptor Receptors distribution distributed throughout various primarily in body tissues epithelial cells and hepatocytes Antiviral effects Antiviral effects Adverse events of Type I IFNs treatment Peg‐Intron • Flu-like symptoms PegIFN‐2a Type III IFNs Potentially fewer • Haematologic IFN omega Peg‐IFN‐ adverse events disorders IFN‐alfa‐2b XL lambda than with type I • Psychiatric Belerofon (Peg‐rIL‐29) interferons symptoms Albuferon Locteron Adapted from 1. Marcello T et al. Gastroenterology 2006;131:1887–98; 2. Muir AJ et al. 2009 AASLD. Abstract 1591; 3. O'Brien TR. Nat Genet. 2009;41:1048–50. PEG-Interferon alfa / Ribavirin Approval studies: efficacy Approval study (n=1530) Approval study (n=1121) Therapy: IFN vs. PEG-IFN alfa 2b Therapy: IFN vs. PEG-IFN alfa 2a 1,0/1,5µg/kgKG -

Development of Novel Antiviral Therapies for Hepatitis C Virus Kai Lin** (Novartis Institutes for Biomedical Research, Inc
VIROLOGICA SINICA, August 2010, 25 (4):246-266 DOI 10.1007/s12250-010-3140-2 © Wuhan Institute of Virology, CAS and Springer-Verlag Berlin Heidelberg 2010 Development of Novel Antiviral Therapies for Hepatitis C Virus Kai Lin** (Novartis Institutes for BioMedical Research, Inc. Cambridge, Massachusetts 02139, USA) Abstract: Over 170 million people worldwide are infected with hepatitis C virus (HCV), a major cause of liver diseases. Current interferon-based therapy is of limited efficacy and has significant side effects and more effective and better tolerated therapies are urgently needed. HCV is a positive, single-stranded RNA virus with a 9.6 kb genome that encodes ten viral proteins. Among them, the NS3 protease and the NS5B polymerase are essential for viral replication and have been the main focus of drug discovery efforts. Aided by structure-based drug design, potent and specific inhibitors of NS3 and NS5B have been identified, some of which are in late stage clinical trials and may significantly improve current HCV treatment. Inhibitors of other viral targets such as NS5A are also being pursued. However, HCV is an RNA virus characterized by high replication and mutation rates and consequently, resistance emerges quickly in patients treated with specific antivirals as monotherapy. A complementary approach is to target host factors such as cyclophilins that are also essential for viral replication and may present a higher genetic barrier to resistance. Combinations of these inhibitors of different mechanism are likely to become the essential components of future HCV therapies in order to maximize antiviral efficacy and prevent the emergence of resistance. -
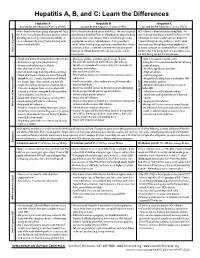
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Active Peptic Ulcer Disease in Patients with Hepatitis C Virus-Related Cirrhosis: the Role of Helicobacter Pylori Infection and Portal Hypertensive Gastropathy
dore.qxd 7/19/2004 11:24 AM Page 521 View metadata, citation and similar papers at core.ac.uk ORIGINAL ARTICLE brought to you by CORE provided by Crossref Active peptic ulcer disease in patients with hepatitis C virus-related cirrhosis: The role of Helicobacter pylori infection and portal hypertensive gastropathy Maria Pina Dore MD PhD, Daniela Mura MD, Stefania Deledda MD, Emmanouil Maragkoudakis MD, Antonella Pironti MD, Giuseppe Realdi MD MP Dore, D Mura, S Deledda, E Maragkoudakis, Ulcère gastroduodénal évolutif chez les A Pironti, G Realdi. Active peptic ulcer disease in patients patients atteints de cirrhose liée au HCV : Le with hepatitis C virus-related cirrhosis: The role of Helicobacter pylori infection and portal hypertensive rôle de l’infection à Helicobacter pylori et de la gastropathy. Can J Gastroenterol 2004;18(8):521-524. gastropathie liée à l’hypertension portale BACKGROUND & AIM: The relationship between Helicobacter HISTORIQUE ET BUT : Le lien entre l’infection à Helicobacter pylori pylori infection and peptic ulcer disease in cirrhosis remains contro- et l’ulcère gastroduodénal dans la cirrhose reste controversé. Le but de la versial. The purpose of the present study was to investigate the role of présente étude est de vérifier le rôle de l’infection à H. pylori et de la gas- H pylori infection and portal hypertension gastropathy in the preva- tropathie liée à l’hypertension portale dans la prévalence de l’ulcère gas- lence of active peptic ulcer among dyspeptic patients with compen- troduodénal évolutif chez les patients dyspeptiques souffrant d’une sated hepatitis C virus (HCV)-related cirrhosis. -

Reviews in Basic and Clinical Gastroenterology
GASTROENTEROLOGY 2010;138:447–462 REVIEWS IN BASIC AND CLINICAL GASTROENTEROLOGY REVIEWS IN BASIC AND CLINICAL John P. Lynch and David C. Metz, Section Editors GASTROENTEROLOGY Resistance to Direct Antiviral Agents in Patients With Hepatitis C Virus Infection CHRISTOPH SARRAZIN and STEFAN ZEUZEM J. W. Goethe-University Hospital, Medizinische Klinik 1, Frankfurt am Main, Germany Chronic hepatitis C virus (HCV) infection is one of agents that are also named specific, targeted antiviral the major causes of cirrhosis, hepatocellular carci- therapies (STAT-C) for HCV infection are in phase 1–3 noma, and liver failure that leads to transplantation. trials. We review resistance to DAA agents, focusing on The current standard treatment, a combination of compounds in development such as reagents that target pegylated interferon alfa and ribavirin, eradicates the the HCV nonstructural (NS)3 protease, the NS5A pro- virus in only about 50% of patients. Directly acting tein, and the RNA-dependent RNA polymerase NS5B. We antiviral (DAA) agents, which inhibit HCV replica- also discuss indirect inhibitors or compounds that in- tion, are in phase 1, 2, and 3 trials; these include hibit HCV replication by not yet completely resolved reagents that target the nonstructural (NS)3 protease, mechanisms, such as cyclophilin inhibitors, nitazox- the NS5A protein, the RNA-dependent RNA-polymer- anide, and silibinin (Table 1, Figure 1). ase NS5B, as well as compounds that directly inhibit HCV replication through interaction with host cell Parameters That Affect Resistance proteins. Because of the high genetic heterogeneity of Heterogeneity of HCV HCV and its rapid replication, monotherapy with HCV has a high rate of turnover; its half-life was DAA agents poses a high risk for selection of resistant estimated to be only 2–5 hours, with the production and variants. -

Understanding Human Astrovirus from Pathogenesis to Treatment
University of Tennessee Health Science Center UTHSC Digital Commons Theses and Dissertations (ETD) College of Graduate Health Sciences 6-2020 Understanding Human Astrovirus from Pathogenesis to Treatment Virginia Hargest University of Tennessee Health Science Center Follow this and additional works at: https://dc.uthsc.edu/dissertations Part of the Diseases Commons, Medical Sciences Commons, and the Viruses Commons Recommended Citation Hargest, Virginia (0000-0003-3883-1232), "Understanding Human Astrovirus from Pathogenesis to Treatment" (2020). Theses and Dissertations (ETD). Paper 523. http://dx.doi.org/10.21007/ etd.cghs.2020.0507. This Dissertation is brought to you for free and open access by the College of Graduate Health Sciences at UTHSC Digital Commons. It has been accepted for inclusion in Theses and Dissertations (ETD) by an authorized administrator of UTHSC Digital Commons. For more information, please contact [email protected]. Understanding Human Astrovirus from Pathogenesis to Treatment Abstract While human astroviruses (HAstV) were discovered nearly 45 years ago, these small positive-sense RNA viruses remain critically understudied. These studies provide fundamental new research on astrovirus pathogenesis and disruption of the gut epithelium by induction of epithelial-mesenchymal transition (EMT) following astrovirus infection. Here we characterize HAstV-induced EMT as an upregulation of SNAI1 and VIM with a down regulation of CDH1 and OCLN, loss of cell-cell junctions most notably at 18 hours post-infection (hpi), and loss of cellular polarity by 24 hpi. While active transforming growth factor- (TGF-) increases during HAstV infection, inhibition of TGF- signaling does not hinder EMT induction. However, HAstV-induced EMT does require active viral replication. -

Hepatitis C 2005 Clinical Guidelines Summary of The: New York State Department of Health Clinical Guidelines for the Medical Management of Hepatitis C
Hepatitis C 2005 Clinical Guidelines Summary of the: New York State Department of Health Clinical Guidelines for the Medical Management of Hepatitis C Inside: Key Features of Viral Hepatitis A,B and C 1 Natural Course of HCV Infection 2 Persons at Risk for HCV Infection 3 Sources of HCV Infection 4 Counseling Prior To Testing 5 Screening for HCV Algorithm 6 Laboratory Testing for HCV 7 Interpretation of HCV Test Results 8 Post Exposure Screening for HCV 9 Counseling After Testing 10 Treating HCV Patients 11 Medical Management of HCV Positive Patients 12 References and Internet Resources 13 Hepatitis C Virus The Hidden Epidemic The Burden of HCV • 3 million Americans are chronically infected with the • 8-10,000 deaths a year are caused by HCV. Hepatitis C virus (HCV). • HCV is the leading cause for liver transplants and • 342,000 New Yorkers are estimated to be infected chronic liver disease. with HCV. • HCV deaths will increase four-fold to 38,000, by • A majority of the people infected with HCV do not the year 2010. know they have it. • Years of life lost to Hepatitis C (2001-2019) • Thousands of people go undetected each year—due 3.1 million years to inadequate risk assessment, under-screening and confusion about the use of diagnostic tests. • Cost of premature disability and death (2010-2109) $75.5 billion Hepatitis C Virus • Direct medical costs in absentee losses due to Hepatitis C $750 million/ year • HCV is a blood-borne disease transmitted by • Total medical expenditures for persons with HCV blood-to-blood contact. -

Hepatitis C Virus Infection and Human Pancreatic ß-Cell Dysfunction
Pathophysiology/Complications BRIEF REPORT Hepatitis C Virus Infection and Human Pancreatic -Cell Dysfunction 1 1 MATILDE MASINI, MD SILVIA DEL GUERRA, PHD showing the characteristic insulin gran- 2 1 DANIELA CAMPANI, MD MARCO BUGLIANI, PHD ules and normally preserved mitochon- 3 1 UGO BOGGI, MD SCILLA TORRI, PHD  2 1 dria. In -cells from HCV-positive MICHELE MENICAGLI, MD STEFANO DEL PRATO, MD 1 3 pancreases, the presence of virus-like par- NICOLA FUNEL, MD FRANCO MOSCA, MD 1 4 ticles was observed, mainly close to the MARIA POLLERA, MD FRANCO FILIPPONI, MD 1 1 membranes of Golgi apparatus, which, in ROBERTO LUPI, PHD PIERO MARCHETTI, MD, PHD turn, appeared hyperplastic and dilated (Fig. 1D). The mitochondria appeared round-shaped with dispersed matrix and fragmented cristae (Fig. 1D). Additional Ϯ 2 any patients with chronic hepati- and 2 women, BMI 25.8 1.6 kg/m ) -cell changes were observed at the Ϯ tis C virus (HCV) develop type 2 and 10 HCV-negative (age 67 9 years, level of rough endoplasmic reticulum, Ϯ diabetes (1). This prevalence is 6 men and 4 women, BMI 26.8 2.0 which showed long and dilated tubular M 2 much higher than that observed in the kg/m ) donors were harvested and stud- membranes, with numerous electron- general population and in patients with ied with the approval of our local ethics dense ribosomes bound to the latter (not other chronic liver diseases such as hepa- committee. Histological studies were shown). These morphological changes titis B virus, alcoholic liver disease, and performed by immunohistochemistry were accompanied by reduced in vitro primary biliary cirrhosis. -

2019 Icar Program & Abstracts Book
Hosted by the International Society for Antiviral Research (ISAR) ND International Conference 32on Antiviral Research (ICAR) Baltimore MARYLAND PROGRAM and USA Hyatt Regency BALTIMORE ABSTRACTS May 12-15 2019 ND TABLE OF International Conference CONTENTS 32on Antiviral Research (ICAR) Daily Schedule . .3 Organization . 4 Contributors . 5 Keynotes & Networking . 6 Schedule at a Glance . 7 ISAR Awardees . 10 The 2019 Chu Family Foundation Scholarship Awardees . 15 Speaker Biographies . 17 Program Schedule . .25 Posters . 37 Abstracts . 53 Author Index . 130 PROGRAM and ABSTRACTS of the 32nd International Conference on Antiviral Research (ICAR) 2 ND DAILY International Conference SCHEDULE 32on Antiviral Research (ICAR) SUNDAY, MAY 12, 2019 › Women in Science Roundtable › Welcome and Keynote Lectures › Antonín Holý Memorial Award Lecture › Influenza Symposium › Opening Reception MONDAY, MAY 13, 2019 › Women in Science Award Lecture › Emerging Virus Symposium › Short Presentations 1 › Poster Session 1 › Retrovirus Symposium › ISAR Award of Excellence Presentation › PechaKucha Event with Introduction of First Time Attendees TUESDAY, MAY 14, 2019 › What’s New in Antiviral Research 1 › Short Presentations 2 & 3 › ISAR Award for Outstanding Contributions to the Society Presentation › Career Development Panel › William Prusoff Young Investigator Award Lecture › Medicinal Chemistry Symposium › Poster Session 2 › Networking Reception WEDNESDAY, MAY 15, 2019 › Gertrude Elion Memorial Award Lecture › What’s New in Antiviral Research 2 › Shotgun Oral -

Vaccinations for Adults with Chronic Liver Disease Or Infection
Vaccinations for Adults with Chronic Liver Disease or Infection This table shows which vaccinations you should have to protect your health if you have chronic hepatitis B or C infection or chronic liver disease (e.g., cirrhosis). Make sure you and your healthcare provider keep your vaccinations up to date. Vaccine Do you need it? Hepatitis A Yes! Your chronic liver disease or infection puts you at risk for serious complications if you get infected with the (HepA) hepatitis A virus. If you’ve never been vaccinated against hepatitis A, you need 2 doses of this vaccine, usually spaced 6–18 months apart. Hepatitis B Yes! If you already have chronic hepatitis B infection, you won’t need hepatitis B vaccine. However, if you have (HepB) hepatitis C or other causes of chronic liver disease, you do need hepatitis B vaccine. The vaccine is given in 2 or 3 doses, depending on the brand. Ask your healthcare provider if you need screening blood tests for hepatitis B. Hib (Haemophilus Maybe. Some adults with certain high-risk conditions, for example, lack of a functioning spleen, need vaccination influenzae type b) with Hib. Talk to your healthcare provider to find out if you need this vaccine. Human Yes! You should get this vaccine if you are age 26 years or younger. Adults age 27 through 45 may also be vacci- papillomavirus nated against HPV after a discussion with their healthcare provider. The vaccine is usually given in 3 doses over a (HPV) 6-month period. Influenza Yes! You need a dose every fall (or winter) for your protection and for the protection of others around you. -

PDF Full-Text
Review Article Chronic Hepatitis C Infection in Children: Current Treatment and New Therapies Andrew Lee1,2*, Jeremy Rajanayagam1,3 and Mona Abdel-Hady1 1Birmingham Children’s Hospital, Liver Unit, Birmingham, United Kingdom; 2The University of Queensland, School of Medicine, Brisbane, Australia; 3University of Birmingham, School of Infection and Immunity, Birmingham, United Kingdom Abstract remains the most common method of infection in the developing world.1,2 The overall rate of spontaneous viral Viral hepatitis C is responsible for a large burden of disease clearance following childhood infection is low, with the worldwide. Treatment of hepatitis C infection is currently majority of children developing chronic hepatitis C (CHC) undergoing a revolution with the development of new direct (54–86%).2 The clinical course of CHC in children is usually acting antivirals that offer higher cure rates and fewer side silent, with mildly abnormal liver function tests (LFTs) and effects than other medications currently available. Treatment minimal inflammation and fibrosis on histology.2–4 options for children, although well-defined and evidence- Nevertheless, fibrosis tends to progress with time, culminat- based, are limited relative to adults as there are few trials ing in cirrhosis in 5–10% or hepatocellular carcinoma (HCC) regarding the use of these newly developed agents in in 2–5% in adulthood.2,3 Thus, continued efforts to effectively children. With so much optimism in the development of novel treat children and reduce the long-term health and social therapeutic options for hepatitis C, it is timely to review and consequences in pediatric CHC are justified. summarize the current standard of care treatment and indications for treatment of chronic hepatitis C in children. -

Polymerase Inhibitors
New Therapeutic Strategies: Polymerase Inhibitors 6th Paris Hepatitis Conference 14th - 15th January, 2013 Stefan Zeuzem Goethe University Hospital Frankfurt, Germany Direct antiviral targets C E1 E2 p7 NS2 NS3 NS4A NS4B NS5A NS5B NS5A NS3 Bifunctional NS5B RNA-dependent protease / helicase RNA polymerase Antiviral Activity of DAAs Vary Among and Within Classes 3-14 day monotherapy in genotype 1 patients NS3 protease inhibitors NS5A inhibitors non-nucleoside inhibitors nucleos/tide inhibitors Median or Mean HCV RNA Decline (log IU/mL) Median or Mean HCV RNA BCP, boceprevir; TVP, telaprevir. Sarrazin C, Zeuzem S. Gastroenterology. 2010;138:447-62. Characteristics of DAAs and HTAs Efficacy Genotype Barrier to independency resistance NS3/4A (protease inhibitors) +++ ++ + - ++ NS5A +++ + - ++ + - ++ NS5B (nucleosides) + - +++ +++ +++ NS5B (non-nucleosides) + - ++ + + Cyclophilin Inhibitors ++ +++ +++ Nucleosidic polymerase inhibitors • RG-7128 (Mericitabine): moderate efficacy, safe • GS-7977 (Sofosbuvir): high efficacy, safe • Alios-2200 = VX135: high efficacy, early phase 2 • NM-283 (Valopicitabine): low efficacy, GI toxicity > • R-1626: moderate efficacy, lymphopenia > • INX-189 = BMS-986094: high efficacy, cardiac tox. > • IDX-184: moderate efficacy, on hold • PSI-938: high efficacy, hepatotoxicity > Nucleosidic polymerase inhibitors • RG-7128 (Mericitabine): moderate efficacy, safe • GS-7977 (Sofosbuvir): high efficacy, safe • Alios-2200 = VX135: high efficacy, early phase 2 • NM-283 (Valopicitabine): low efficacy, GI toxicity > • R-1626: moderate efficacy, lymphopenia > • INX-189 = BMS-986094: high efficacy, cardiac tox. > • IDX-184: moderate efficacy, on hold • PSI-938: high efficacy, hepatotoxicity > PROPEL study: Efficacy and safety of MCB + PR in G1/4 treatment-naive patients . Mericitabine nucleoside analog MCB 500 mg 12 wk (RVR-guided) polymerase inhibitor 100 MCB 1000 mg 8 wk (RVR-guided) MCB 1000 mg 12 wk (RVR-guided) .