Aldehydes, Screening 2539
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Octyl Alcohol F Oxo Alcohols
Octyl alcohol F Oxo alcohols Trade name: Octyl alcohol F Chemical name: Distillation residue, by-products from the production of 2-ethylhexan-1-ol. CN: 3824 90 97 CAS: 68609-68-7 Properties Use Octyl alcohol F is a liquid whose colour varies from yellow, Octyl alcohol F is used as a flotation agent. through brown-yellow to greenish, with a characteristic odour. Product classification Octyl alcohol F is not classified as a hazardous material according to RID/ADR. Physical and chemical properties Parameter Business unit Value Test methods 2-ethylhexanol, not more than % m/m 30 ZAK’s internal rmethod High-molecular compounds >C8, not less than % m/m 70 ZAK’s internal rmethod oxoplast.pl Manufacturer: Grupa Azoty Zakłady Azotowe Kędzierzyn S.A. Isobutyraldehyde Trade name: Isobutyraldehyde Chemical name: 2-methylpropanal, isobutyraldehyde, isobutanal CN: 2912 19 00 CAS: 78-84-2 Chemical formula: (CH3)2CHCHO Properties Use Isobutyraldehyde is a transparent, colourless liquid with a char- Isobutyraldehyde is used as a raw material for producing acteristic odour. alcohols, acids, amines, and esters. It is used in processes of manufacturing plasticizers, pharmaceutical products, plant Product classification protection agents, synthetic resins, fragrances, solvents and all sorts of additives used in many branches of industry Isobutyraldehyde is classified as a hazardous material (antioxidants, wetting agents, perfume ingredients, improvers). according to RID/ADR. - RID KI. 3, packing group II - ADR KI. 3, packing group II Physical and chemical properties Parameter Business unit Value Test methods Colour, not more than Pt-Co 15 ISO 6271 Acid number, not more than mg KOH/g 2 ZAK’s internal rmethod N-butyraldehyde, not more than % m/m 0,2* ZAK’s internal rmethod Water, not more than % m/m 1,5 ISO 760 Isobutyraldehyde, not less than % m/m 99,5* ZAK’s internal rmethod * the values do not take into account water content in the product oxoplast.pl Manufacturer: Grupa Azoty Zakłady Azotowe Kędzierzyn S.A. -

Review Article Di-2-Ethylhexylphthalate May Be a Natural Product, Rather Than a Pollutant
Hindawi Journal of Chemistry Volume 2018, Article ID 6040814, 7 pages https://doi.org/10.1155/2018/6040814 Review Article Di-2-ethylhexylphthalate May Be a Natural Product, Rather than a Pollutant Aurelio Ortiz and Estibaliz Sansinenea Facultad de Ciencias Qu´ımicas, Beneme´rita Universidad Auto´noma de Puebla, C.P. 72570, Puebla, PUE, Mexico Correspondence should be addressed to Estibaliz Sansinenea; [email protected] Received 28 June 2018; Revised 22 August 2018; Accepted 3 September 2018; Published 26 September 2018 Academic Editor: Qizhen Du Copyright © 2018 Aurelio Ortiz and Estibaliz Sansinenea. )is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Di-2-ethylhexylphtalate is an ester of phthalic acid that has been used as plasticizer in many materials. Due to the extended use, it has been persistently found in different environments being classified as a pollutant with some risks for human health. However, in the last years, it has been found that this compound is produced by plants or microorganisms like bacteria or fungi. )is finding opened a serious debate about the origin of this compound and questioned if it is a real pollutant or a natural metabolite with some biological activities that could help us in several ways. )is review tries to give some data of the different points of view about this question. 1. Introduction presence of these compounds in the environment, many reactions have been reported to degrade and transform these Phthalate compounds are colorless liquid chemicals that compounds. -

Final Scope of the Risk Evaluation for Formaldehyde CASRN 50-00-0
EPA Document# EPA-740-R-20-014 August 2020 United States Office of Chemical Safety and Environmental Protection Agency Pollution Prevention Final Scope of the Risk Evaluation for Formaldehyde CASRN 50-00-0 August 2020 TABLE OF CONTENTS ACKNOWLEDGEMENTS ......................................................................................................................6 ABBREVIATIONS AND ACRONYMS ..................................................................................................7 EXECUTIVE SUMMARY .....................................................................................................................11 1 INTRODUCTION ............................................................................................................................14 2 SCOPE OF THE EVALUATION ...................................................................................................14 2.1 Reasonably Available Information ..............................................................................................14 Search of Gray Literature ...................................................................................................... 15 Search of Literature from Publicly Available Databases (Peer-reviewed Literature) ........... 16 Search of TSCA Submissions ................................................................................................ 24 2.2 Conditions of Use ........................................................................................................................25 Conditions of Use -

Purification and Properties of the Inducible Coenzyme A-Linked Butyraldehyde Dehydrogenase from Clostridium Acetobutylicum NEIL R
JOURNAL OF BACTERIOLOGY, JUlY 1988, p. 2971-2976 Vol. 170, No. 7 0021-9193/88/072971-06$02.00/0 Copyright © 1988, American Society for Microbiology Purification and Properties of the Inducible Coenzyme A-Linked Butyraldehyde Dehydrogenase from Clostridium acetobutylicum NEIL R. PALOSAARI* AND PALMER ROGERS Department of Microbiology, University of Minnesota, Minneapolis, Minnesota 55455 Received 13 November 1987/Accepted 25 March 1988 The coenzyme A (CoA)-linked butyraldehyde dehydrogenase (BAD) from Clostridium acetobutylicum was characterized and purified to homogeneity. The enzyme was induced over 200-fold, coincident with a shift from an acidogenic to a solventogenic fermentation, during batch culture growth. The increase in enzyme activity was found to require new protein synthesis since induction was blocked by the addition of rifampin and antibody against the purified enzyme showed the appearance of enzyme antigen beginning at the shift of the fermentation and increasing coordinately with the increase in enzyme specific activity. The CoA-linked acetaldehyde dehydrogenase was copurified with BAD during an 89-fold purification, indicating that one enzyme accounts for the synthesis of the two aldehyde intermediates for both butanol and ethanol production. Butanol dehydrogenase activity was clearly separate from the BAD enzyme activity on TEAE cellulose. A molecular weight of 115,000 was determined for the native enzyme, and the enzyme subunit had a molecular weight of 56,000 indicating that the active form is a homodimer. Kinetic constants were determined in both the forward and reverse directions. In the reverse direction both the V,ax and the apparent affinity for butyraldehyde and caproaldehyde were significantly greater than they were for acetaldehyde, while in the forward direction, the Vmax for butyryl-CoA was fivefold that for acetyl-CoA. -

Sids Initial Assessment Profile
SIAM 31, 20-22 October 2010 BIAC/ICCA SIDS INITIAL ASSESSMENT PROFILE CAS No. 123-05-7 Chemical Name 2-Ethylhexaldehyde Structural Formula O=CH-CH(CH2-CH3)-CH2-CH2-CH2-CH3 SUMMARY CONCLUSIONS OF THE SIAR Analogue Rationale Several of the health endpoints for 2-ethylhexaldehyde make use of data from 2-ethylhexanol and 2-ethylhexanoic acid experiments. The logic for this “metabolic series” approach includes the metabolism of alcohols (2-ethylhexanol) proceeding via a readily reversible reaction with alcohol dehydrogenase(s) to rapidly form the respective aldehydes (2-ethylhexaldehyde). The aldehydes are short-lived due to the enzymatic activity of aldehyde dehydrogenase that forms the respective organic acids (2-ethylhexanoic acid). Assuming 2-ethylhexaldehyde behaves as other aldehydes, direct exposure to 2-ethylhexaldehyde will result in the rapid formation of both 2 -ethylhexanol and 2-ethylhexanoic acid. The metabolic processes of these two chemicals are well characterized. Toxicity data from studies conducted with 2-ethylhexanol and 2-ethylhexanoic acid have been used to identify the hazards associated with systemic exposure to 2 -ethylhexaldehyde and therefore are useful in identifying hazards associated with 2-ethylhexaldehyde systemic exposures. Structural analogues are used to address several Environmental Hazard endpoints: 2-methyl propionaldehyde (CAS No.78-84-2), 2-ethyl butyraldehyde (CAS No.97-96-1), 2-methyl valeraldehyde (CAS No. 123-15-9), valeraldehyde (CAS No. 110-62-3), hexaldehyde (CAS No.66-25-1), and octylaldehyde (CAS No. 124-13-0). Physical-Chemical Properties 2-Ethylhexaldehyde is a liquid at standard temperature and pressure, with a boiling point of 163 ºC and a melting (freezing) point of <–100 ºC. -

Kinetics of Oxidation of Formaldehyde, Acetaldehyde, Propionaldehyde & Butyraldehyde by Ditelluratocuprate(III) in Alkaline Medium
r Jndlan Journal of Chemistry Vol. 17A, January 1979,pp. 48·51 Kinetics of Oxidation of Formaldehyde, Acetaldehyde, Propionaldehyde & Butyraldehyde by Ditelluratocuprate(III) in Alkaline Medium C. P. MURTHY, B. SETHURAM & T. NAVANEETH RAO· Department of Chemistry, Osmania University, Hyderabad 500007 Received 8 June 1978; accepted 25 July 1978 Kinetics of oxidation oJ formaldehyde, acetaldehyde, proplonaldehyde and n-butyraldehyde by potasstum ditelluratocuprate(III) has been studied in alkaline medium spectrophoto- :metrically. The order in [aldeh;de] and [Cu(III)) are found to be one each and rates decreased with increase in [tellurate] and increase in [OH-]. There is no effect of addition of salts like Na, SO. and KNOa• The products of oxidation are identified as corresponding carboxylic acids. Under the experimental conditions the :monotelluratocuprate(III) species is assu:med as the active species. The ther:modynamic para:meters are also reported and a plausible mechanism has been suggested. SE of Cu(III) as an oxidizing agent is well corrections made for any self-decomposition of known in analytical chemistry in the estimation Cu (III) during the reaction. U of sugars-, glycerols-, amino acids", proteinss, carboxylic acids", carbonyl compounds" and alcohols? Results The presence of Cu(III) as intermediate was also Under the conditions [Cu(III)] ~ [aldehyde] the reported in some Cu(II)-catalysed oxidation reactions plots of log (absorbance) versus time were linear by peroxydisulphate" and vanadium (V)9. The kine- (Fig. lA), indicating the order in [Cu(III)J to be tics of decomposition and formation of Cu(III) unity. From the slopes of the above plots the diperiodate and ditellurate complexes were also pseudo-first order rate constants (k') were evaluated. -

Oxidation of Isobutyraldehyde
Indlan Journal of Chemlstry Vol. 15A, August 1977, pp. 705-708 Kinetics & Mechanism of Cr(VI) Oxidation of Isobutyraldehyde A. A. BHALEKAR, R. SHANKER & G. V. BAKORE Department of Chemistry, University of Udaipur, Udaipur 313001 Received 16 August 1976; accepted 26 March 1977 Cr(VI) oxidation of isobutyraldehyde has been found to take place through the following mechanism: (i) 70% of isobutyraldehyde oxidation occurs via the hydrated form and (ii) 30% of isobutyraldebyde undergoes oxidation via enol intermediate. The reaction follows the rate law: ~d[Cr(VI)]/dt = k'kE[Aldehyde][H+J[HCrO,]/(kA+k'[H+][HCrO-])+k"Kh[Aldehyde][H+][HCrO&] where kh, k', k', kE and kA are equilibrium constant for hydration of aldehyde, rate of oxidation of enol, rate of oxidation of ketone, rate of enolization and the rate of ketonization, respectively. kE obtained from the oxidation of isobutyraldehyde by V(V) under identical conditions as used in chromic acid oxidation, is of the same order. ARNARD and Karayannis! while investigating Results and Discussion chromic acid oxidation of propionaldehyde B and t.-butyraldehyde made some interesting Stoichiometry and identification oj products - These observations. They found that propionaldehyde ~ere carried out in aq. solution keeping [Cr(VI)] reduced 170% of the expected amount of chromic In large excess over [isobutyraldehyde]. It was acid and that besides propionic acid, acetic acid found that 0·94 mole (2·8 equivalents) of C r(VI) was also produced while n-butyraldehyde consumed was consumed per mole of the aldehyde. Under 190% of the theoretical amount of chromic acid stoichiometric conditions, the excess Cr(VI) was and that both propionic acid and to a lesser extent reduced with Fe (II) ions and the solution steam- acetic acid were produced. -

Food and Drugs
21 Part 170 to 199 Revised as of April 1, 2001 Food and Drugs Containing a codification of documents of general applicability and future effect As of April 1, 2001 With Ancillaries Published by Office of the Federal Register National Archives and Records Administration A Special Edition of the Federal Register VerDate 11<MAY>2000 11:38 Apr 16, 2001 Jkt 194064 PO 00000 Frm 00001 Fmt 8091 Sfmt 8091 Y:\SGML\194064F.XXX pfrm02 PsN: 194064F U.S. GOVERNMENT PRINTING OFFICE WASHINGTON : 2001 For sale by the Superintendent of Documents, U.S. Government Printing Office Internet: bookstore.gpo.gov Phone: (202) 512-1800 Tax: (202) 512-2250 Mail Stop: SSOP, Washington, DC 20402–0001 VerDate 11<MAY>2000 11:38 Apr 16, 2001 Jkt 194064 PO 00000 Frm 00002 Fmt 8092 Sfmt 8092 Y:\SGML\194064F.XXX pfrm02 PsN: 194064F Table of Contents Page Explanation ................................................................................................ v Title 21: Chapter I—Food and Drug Administration, Department of Health and Human Services (Continued) ................................................. 3 Finding Aids: Material Approved for Incorporation by Reference ............................ 573 Table of CFR Titles and Chapters ....................................................... 587 Alphabetical List of Agencies Appearing in the CFR ......................... 605 Redesignation Table ............................................................................ 615 List of CFR Sections Affected ............................................................. 617 -

Butyraldehyde Casrn: 123-72-8 Unii: H21352682a
BUTYRALDEHYDE CASRN: 123-72-8 UNII: H21352682A FULL RECORD DISPLAY Displays all fields in the record. For other data, click on the Table of Contents Human Health Effects: Human Toxicity Excerpts: /HUMAN EXPOSURE STUDIES/ Three Asian subjects who reported experiencing severe facial flushing in response to ethanol ingestion were subjects of patch testing to aliphatic alcohols and aldehydes. An aqueous suspension of 75% (v/v) of each alcohol and aldehyde was prepared and 25 uL was used to saturate ashless grade filter paper squares which were then placed on the forearm of each subject. Patches were covered with Parafilm and left in place for 5 minutes when the patches were removed and the area gently blotted. Sites showing erythema during the next 60 minutes were considered positive. All three subjects displayed positive responses to ethyl, propyl, butyl, and pentyl alcohols. Intense positive reactions, with variable amounts of edema, were observed for all the aldehydes tested (valeraldehyde as well as acetaldehyde, propionaldehyde, and butyraldehyde). [United Nations Environment Programme: Screening Information Data Sheets on n-Valeraldehyde (110-62-3) (October 2005) Available from, as of January 15, 2009: http://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html] **PEER REVIEWED** [United Nations Environment Programme: Screening Information Data Sheets on nValeraldehyde (110623) (October 2005) Available from, as of January 15, 2009: http://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html] **PEER REVIEWED** /SIGNS AND SYMPTOMS/ May act as irritant, /SRP: CNS depressant/ ...[Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. -

The Impact of Ethanol and Iso-Butanol Blends on Gaseous and Particulate
Energy 82 (2015) 168e179 Contents lists available at ScienceDirect Energy journal homepage: www.elsevier.com/locate/energy The impact of ethanol and iso-butanol blends on gaseous and particulate emissions from two passenger cars equipped with spray-guided and wall-guided direct injection SI (spark ignition) engines * Georgios Karavalakis a, b, , Daniel Short a, b, Diep Vu a, b, Robert L. Russell a, Akua Asa-Awuku a, b, Heejung Jung a, c, Kent C. Johnson a, b, Thomas D. Durbin a, b a University of California, Bourns College of Engineering, Center for Environmental Research and Technology (CE-CERT), 1084 Columbia Avenue, Riverside, CA 92507, USA b Department of Chemical and Environmental Engineering, Bourns College of Engineering, University of California, Riverside, CA 92521, USA c Department of Mechanical Engineering, Bourns College of Engineering, University of California, Riverside, CA 92521, USA article info abstract Article history: We examined the effects of different ethanol and iso-butanol blends on the gaseous and particulate Received 2 August 2014 emissions from two passenger cars equipped with spark ignition direct injection engines and with one Received in revised form spray-guided and one wall-guided configuration. Both vehicles were tested over triplicate FTP (Federal 3 December 2014 Test Procedure) and UC (Unified Cycles) using a chassis dynamometer. Emissions of THC (total hydro- Accepted 8 January 2015 carbons), NMHC (non-methane hydrocarbons), and CO (carbon monoxide) reduced with increasing Available online 7 February 2015 oxygen content in the blend for some of the vehicle/fuel combinations, whereas NOx (nitrogen oxide) emissions did not show strong fuel effects. -
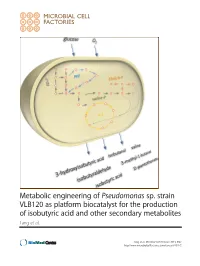
View of Bacterial Expression Systems for Heterologous 74
Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production of isobutyric acid and other secondary metabolites Lang et al. Lang et al. Microbial Cell Factories 2014, 13:2 http://www.microbialcellfactories.com/content/13/1/2 Lang et al. Microbial Cell Factories 2014, 13:2 http://www.microbialcellfactories.com/content/13/1/2 RESEARCH Open Access Metabolic engineering of Pseudomonas sp. strain VLB120 as platform biocatalyst for the production of isobutyric acid and other secondary metabolites Karsten Lang, Jessica Zierow, Katja Buehler* and Andreas Schmid Abstract Background: Over the recent years the production of Ehrlich pathway derived chemicals was shown in a variety of hosts such as Escherichia coli, Corynebacterium glutamicum, and yeast. Exemplarily the production of isobutyric acid was demonstrated in Escherichia coli with remarkable titers and yields. However, these examples suffer from byproduct formation due to the fermentative growth mode of the respective organism. We aim at establishing a new aerobic, chassis for the synthesis of isobutyric acid and other interesting metabolites using Pseudomonas sp. strain VLB120, an obligate aerobe organism, as host strain. Results: The overexpression of kivd, coding for a 2-ketoacid decarboxylase from Lactococcus lactis in Ps. sp. strain VLB120 enabled for the production of isobutyric acid and isobutanol via the valine synthesis route (Ehrlich pathway). This indicates the existence of chromosomally encoded alcohol and aldehyde dehydrogenases catalyzing -

Method TO-11A
EPA/625/R-96/010b Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air Second Edition Compendium Method TO-11A Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC) [Active Sampling Methodology] Center for Environmental Research Information Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 January 1999 Method TO-11A Acknowledgements This Method was prepared for publication in the Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air, Second Edition (EPA/625/R-96/010b), which was prepared under Contract No. 68-C3-0315, WA No. 3-10, by Midwest Research Institute (MRI), as a subcontractor to Eastern Research Group, Inc. (ERG), and under the sponsorship of the U.S. Environmental Protection Agency (EPA). Justice A. Manning, John O. Burckle, and Scott Hedges, Center for Environmental Research Information (CERI), and Frank F. McElroy, National Exposure Research Laboratory (NERL), all in the EPA Office of Research and Development, were responsible for overseeing the preparation of this method. Additional support was provided by other members of the Compendia Workgroup, which include: • John O. Burckle, U.S. EPA, ORD, Cincinnati, OH • James L. Cheney, Corps of Engineers, Omaha, NB • Michael Davis, U.S. EPA, Region 7, KC, KS • Joseph B. Elkins Jr., U.S. EPA, OAQPS, RTP, NC • Robert G. Lewis, U.S. EPA, NERL, RTP, NC • Justice A. Manning, U.S. EPA, ORD, Cincinnati, OH • William A. McClenny, U.S. EPA, NERL, RTP, NC • Frank F. McElroy, U.S. EPA, NERL, RTP, NC • Heidi Schultz, ERG, Lexington, MA • William T.