Comparative Investigation of Thermal and Structural Behavior in Renewably Sourced Composite Films of Even-Even Nylons (610 and 1010) with Silk Fibroin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
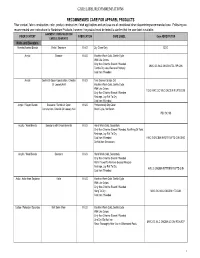
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Choosing the Proper Short Cut Fiber for Your Nonwoven Web
Choosing The Proper Short Cut Fiber for Your Nonwoven Web ABSTRACT You have decided that your web needs a synthetic fiber. There are three important factors that have to be considered: generic type, diameter, and length. In order to make the right choice, it is important to know the chemical and physical characteristics of the numerous man-made fibers, and to understand what is meant by terms such as denier and denier per filament (dpf). PROPERTIES Denier Denier is a property that varies depending on the fiber type. It is defined as the weight in grams of 9,000 meters of fiber. The current standard of denier is 0.05 grams per 450 meters. Yarn is usually made up of numerous filaments. The denier of the yarn divided by its number of filaments is the denier per filament (dpf). Thus, denier per filament is a method of expressing the diameter of a fiber. Obviously, the smaller the denier per filament, the more filaments there are in the yarn. If a fairly closed, tight web is desired, then lower dpf fibers (1.5 or 3.0) are preferred. On the other hand, if high porosity is desired in the web, a larger dpf fiber - perhaps 6.0 or 12.0 - should be chosen. Here are the formulas for converting denier into microns, mils, or decitex: Diameter in microns = 11.89 x (denier / density in grams per milliliter)½ Diameter in mils = diameter in microns x .03937 Decitex = denier x 1.1 The following chart may be helpful. Our stock fibers are listed along with their density and the diameter in denier, micron, mils, and decitex for each: Diameter Generic Type -

Silk, Linen, Leather, Denim, Grass, Cotton, Felt
Silk, linen, leather, denim, grass, cotton, felt. Natural materials keep the integrity of their shape yet hold an impression of the figure that has worn them. Fit-out for Olivia Spencer Bower speaks to the fabrics and forms that we live in. An assortment of garment patterns, building plans and the lifestyles of art heroines provide templates or underlying structures for Emma Fitts’ installation at the Ilam School of Fine Arts gallery. Adopting the layout of a modernist home and featuring a series of fabric hangings and clothing cutouts, Fitts’ work acutely relates to our situated knowledge, the proportions of the body and our experience of space, while also implying much less measurable qualities. Alternative histories that reveal the social relationships and values of a bygone era are incorporated to form a homage of sorts, though these references are evoked to shed light on the present–what it means to live and make work as an artist today. The gallery is physically divided by four large textile works, hung from the ceiling according to the walls shown in architectural plans for 15A Leinster Avenue, Christchurch–the former home of artist Olivia Spencer Bower (1905- 82). These soft walls enable visitors to occupy the space as though wandering through her actual house: living room, kitchen, sunroom, bedroom and studio. Designed in 1969 by architects Cowey and McGregor in the Christchurch style of neo-brutalism, the house was commissioned to accommodate the needs of a female artist living alone. There is an emphasis on form developed in relation to function and it is both refined and compact, manifesting these ideals. -

Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing
machines Article Investigation of the Mechanical Properties of a Carbon Fibre-Reinforced Nylon Filament for 3D Printing Flaviana Calignano 1,* , Massimo Lorusso 2 , Ignanio Roppolo 3 and Paolo Minetola 1 1 Department of Management and Production Engineering, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] 2 Istituto Italiano di Tecnologia, Center for Sustainable Future Technologies IIT@Polito, Corso Trento 21, 10129 Turin, Italy; [email protected] 3 Department of Applied Science and Technology, Politecnico di Torino, Corso Duca degli Abruzzi 24, 10129 Turin, Italy; [email protected] * Correspondence: fl[email protected]; Tel.: +39-011-090-7218 Received: 19 July 2020; Accepted: 2 September 2020; Published: 4 September 2020 Abstract: Additive manufacturing (i.e., 3D printing) has rapidly developed in recent years. In the recent past, many researchers have highlighted the development of in-house filaments for fused filament fabrication (FFF), which can extend the corresponding field of application. Due to the limited mechanical properties and deficient functionality of printed polymer parts, there is a need to develop printable polymer composites that exhibit high performance. This study analyses the actual mechanical characteristics of parts fabricated with a low-cost printer from a carbon fibre-reinforced nylon filament. The results show that the obtained values differ considerably from the values presented in the datasheets of various filament suppliers. Moreover, the hardness and tensile strength are influenced by the building direction, the infill percentage, and the thermal stresses, whereas the resilience is affected only by the building direction. Furthermore, the relationship between the mechanical properties and the filling factor is not linear. -

“Al-Tally” Ascension Journey from an Egyptian Folk Art to International Fashion Trend
مجمة العمارة والفنون العدد العاشر “Al-tally” ascension journey from an Egyptian folk art to international fashion trend Dr. Noha Fawzy Abdel Wahab Lecturer at fashion department -The Higher Institute of Applied Arts Introduction: Tally is a netting fabric embroidered with metal. The embroidery is done by threading wide needles with flat strips of metal about 1/8” wide. The metal may be nickel silver, copper or brass. The netting is made of cotton or linen. The fabric is also called tulle-bi-telli. The patterns formed by this metal embroidery include geometric figures as well as plants, birds, people and camels. Tally has been made in the Asyut region of Upper Egypt since the late 19th century, although the concept of metal embroidery dates to ancient Egypt, as well as other areas of the Middle East, Asia, India and Europe. A very sheer fabric is shown in Ancient Egyptian tomb paintings. The fabric was first imported to the U.S. for the 1893 Chicago. The geometric motifs were well suited to the Art Deco style of the time. Tally is generally black, white or ecru. It is found most often in the form of a shawl, but also seen in small squares, large pieces used as bed canopies and even traditional Egyptian dresses. Tally shawls were made into garments by purchasers, particularly during the 1920s. ملخص البحث: التمي ىو نوع من انواع االتطريز عمى اقمشة منسوجة ويتم ىذا النوع من التطريز عن طريق لضم ابر عريضة بخيوط معدنية مسطحة بسمك 1/8" تصنع ىذه الخيوط من النيكل او الفضة او النحاس.واﻻقمشة المستخدمة في صناعة التمي تكون مصنوعة اما من القطن او الكتان. -

Sea-Silk Based Nanofibers and Their Diameter Prediction THERMAL SCIENCE: Year 2019, Vol
Tian, D., et al.: Sea-Silk Based Nanofibers and Their Diameter Prediction THERMAL SCIENCE: Year 2019, Vol. 23, No. 4, pp. 2253-2256 2253 SEA-SILK BASED NANOFIBERS AND THEIR DIAMETER PREDICTION by * Dan TIAN, Chan-Juan ZHOU, and Ji-Huan HE National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou, China Original scientific paper https://doi.org/10.2298/TSCI1904253T Diameter of sea-silk based nanofibers prepared by electrospinning is closely re- lated to the concentrations of sea-silk solution. A mathematical model is estab- lished according the mass conservation law in fluid mechanics to predict the di- ameter of fibers, and the MATLAB software is used to fit the experiment value. The results show that the fitted equation is quite accurate and efficient for estimating the diameter of fibers with different concentrations. Key words: electrospinning, sea-silk, mathematical model, nanofiber, fiber’s diameter. Introduction Electrospinning is an effective way to prepare nanofibers, it is a fabrication process that uses an electric field to control the deposition of polymer fibers onto a receptor [1-8]. In electrospinning process, the diameter of nanofiber is determined by many factors, like volt- age, viscosity of solution, receptor’s distance, environment temperature, environment humidi- ty, etc. [9, 10]. Nanofiber’s diameter and morphology can also be controlled by additives [11]. The sea silk is one of the oldest natural silks, which has a history of more than 5000 years [12-16], and now we can produce artificial sea silk through Mytilus edulis. To this end, we extract protein from Mytilus edulis and then use it as an additive for electrospinning, this maybe has some effects on the morphology of fibers. -

Nylon Wool Fiber Columns
U.S. Corporate Headquarters Polysciences Europe GmbH Polysciences Asia-Pacific, Inc. 400 Valley Rd. Badener Str. 13 2F-1, 207 DunHua N. Rd. Warrington, PA 18976 69493 Hirschberg an der Taipei, Taiwan 10595 1(800) 523-2575 / (215) 343-6484 Bergstrasse, Germany (886) 2 8712 0600 1(800)343-3291 fax +(49) 6201 845 20 0 (886) 2 8712 2677 fax [email protected] +(49) 6201 845 20 20 fax [email protected] [email protected] TECHNICAL DATA SHEET 425A Page 1 of 2 Nylon Wool Fiber Columns BACKGROUND NYLON WOOL FIBER VS. SHEEP RBC ROSETTING METHODS Researchers have been using nylon wool fiber procedures to separate T-cell and Wong and Mittal (1981)9 did extensive research comparing the methods of Nylon B-cell lymphocytes for more than 20 years. In the early 1970’s M. H. Julius et al Wool Fiber separation and the commonly- used and well-studied sheep RBC (1973),1 Eisen et al (1972),2 and Greaves & Brain (1974)3 described specific (SRBC) rosetting.10,11 Wong and Mittal were interested in isolating B-cells for conditions for the use of Nylon Wool Fiber in columns or plastic straws. These serologic typing of HLA-DR antigen. protocols resulted in yields of 50-90% T-cell recovery and 10-100 fold B-cell depletion. Wong and Mittal concluded that “Due to its simplicity and reliability, nylon wool adherence may be preferred over the SRBC rosette method for the routine pheno- These early researchers found it necessary to scrub or wash their Nylon Wool Fiber typing of B-cells.” Their findings are illustrated in Table 1. -

Silk Cotton Vs. Bombax Vs. Banyan
Ceiba pentandra Kopok tree, Silk-cotton tree Ta Prohm, Cambodia By Isabel Zucker Largest known specimen in Lal Bagh Gardens in Bangalore, India. http://scienceray.com/biology/botany/amazing-trees-from-around-the-world-the-seven-wonder-trees/ Ceiba pentandra Taxonomy • Family: Malvaceae • Sub family: Bombacaceae -Bombax spp. in same family - much online confusion as to which tree is primarily in Ta Praham, Cambodia. • Fig(Moraceae), banyan and kapok trees in Ta Praham • Often referred to as a banyan tree, which is quite confusing. Distribution • Originated in the American tropics, natural and human distribution. • Africa, Asia. – Especially Indonesia and Thailand • Indian ocean islands • Ornamental shade tree • Zone – Humid areas, rainforest, dry areas – Mean annual precipitation 60-224 inches per year – Temperatures ranging from 73-80 unaffected by frost – Elevation from 0-4,500 feet – Dry season ranging from 0-6 months Characteristics • Rapidly growing, deciduous • Reaches height up to 200 feet • Can grow 13 feet per year • Diameter up to 9 feet above buttress – Buttress can extend 10 feet from the trunk and be 10 feet tall • large umbrella-shaped canopies emerge above the forest canopy • http://www.flmnh.ufl.edu/caribarch/ceiba.htm • Home to many animals – Birds, frogs, insects – Flowers open in the evening, pollinated by bats • Epiphytes grow in branches • Compound leaves with 5-8 lance- shaped leaflets 3-8 inches long • Dense clusters of whitish to pink flowers December to February – 3-6 inch long, elliptical fruits. – Seeds of fruit surrounded by dense, cottony fibers. – Fibers almost pure cellulose, buoyant, impervious to water, low thermal conductivity, cannot be spun. -

Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers. Weiying Tao Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1991 Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers. Weiying Tao Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Tao, Weiying, "Interfacial Adhesion in Rayon/Nylon Sheath/Core Composite Fibers." (1991). LSU Historical Dissertations and Theses. 5213. https://digitalcommons.lsu.edu/gradschool_disstheses/5213 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. -

Immersion Dyeing Nylon and Acetate Rayon Using Prosperse Disperse Dyes Please Read Directions Carefully Before Starting
Immersion Dyeing Nylon and Acetate Rayon using PROsperse Disperse Dyes Please read directions carefully before starting. For medium to dark shades, it is recommended that nylon be dyed with acid dyes, because disperse dyes lack acceptable fastness. Acetate rayon can only be dyed with disperse dyes and has acceptable fastness in all depths of shade with the disperse dyes. All Dyeing should be done in a stainless steel or enamelware container only. Never use aluminum pots. Use Pyrex or stainless steel measuring utensils and a large wooden dowel for stirring in the boiling dye bath. Always do test samples before working on a large project. Please Note: These dyes have the potential to stain any sink that is not made of stainless steel or fireclay ceramic. For additional information, visit our web site at www.prochemicalanddye.com. Wear rubber gloves, apron, or old clothes. Utensils used for dyeing should never be used for food preparation. Supplies PROsperse Disperse Dye Citric Acid Crystals or White Distilled Vinegar Synthrapol PRO Dye Activator or Soda Ash Procedure 1. Scour the fabric by machine washing in HOT 140F (60C) water, or by hand in a pot on the stove with 2 tsp (2 gm) PRO Dye Activator or Soda Ash and 2 tsp (2.5 ml) Synthrapol per pound of fabric (454 gm, or 3 to 4 yards of muslin weight fabric). Rinse thoroughly. This step does not add the dye fixative to the fabric; it prepares your fabric for dyeing by removing any dirt, oil or sizing. 2. Dissolve the dye. Thoroughly dissolve the desired amount of dye powder, from the chart below, in 1 cup (250 ml) of boiling water. -

Colaris Digital Textile Printing
ZIMMER AUSTRIA | DIGITAL PRINTING SYSTEMS COLARIS DIGITAL TEXTILE PRINTING HOME TEXTILES APPAREL DECORATION AUTOMOTIVE FLAGS & BANNERS www.zimmer-austria.com 2020.01.15 page 1 CONTENT 1. INNOVATION IS IN OUR DNA 1.1. HISTORIC MILESTONES 3 2. INK CLASSES 2.1. TYPES | PRODUCTS | PROCESS | REQUIREMENTS 4 2.2. TYPES | PRODUCTS | PROCESS | REQUIREMENTS 5 3. PRINT TECHNOLOGY 3.1. PROCESSING DIAGRAM 6 3.2. PROCESS EQUIPMENT 7 4. REACTIVE PRINTING 4.1. GENERAL INFORMATION 8 4.2. EXAMPLE: TERRY TOWEL PRINT PRODUCTION 9 5. ACID PRINTING 5.1. GENERAL INFORMATION 10 5.2. EXAMPLE: UPHOLSTERY PRINT LINE 11 6. DISPERSE / SUBLIMATION PRINTING 6.1. GENERAL INFORMATION 12 6.2. EXAMPLE: PES BLANKET PRINT LINE 13 7. VAT INDANTHRENE® PRINTING 7.1. GENERAL INFORMATION 14 7.2. APPLICATION DIVERSITY 15 8. PIGMENT PRINTING 8.1. GENERAL INFORMATION 16 8.2. APPLICATION DIVERSITY 17 9. CATIONIC PRINTING 9.1. GENERAL INFORMATION 18 10. COLARIS - CHARACTERISTICS AND FEATURES 10.1. COLARIS MODELS 19 11. COLARIS FEATURES AND COMPONENTS 11.1. INTEGRATED MACHINE COMPONENTS 20 11.2. INTEGRATED MACHINE COMPONENTS 21 12. PROCESS EQUIPMENT 12.1. INLINE COMPONENTS 22 12.2. OFFLINE COMPONENTS 23 13. PRINT HEAD 13.1. TECHNOLOGY 24 13.2. RECONDITION CENTER 25 14. ZIMMER TECHNOLOGY & APPLICATION CENTER 14.1. GENERAL INFORMATION 26 14.2. EQUIPMENT & FACILITIES 27 www.zimmer-austria.com 2020.01.15 page 2 1. INNOVATION IS IN OUR DNA 1.1. HISTORIC MILESTONES Vertical Duplex blanket printer from 1951 First commercial rotary screen printer 1958 The broad digital competence of ZIMMER AUSTRIA is based on an innovation introduced more than 4 decades ago. -

Evaluation of the Physical and Diagnostic Nature of Swabs W
EVALUATION OF THE PHYSICAL AND DIAGNOSTIC NATURE OF SWABS W. Nugent, M.D.1, M.J. Russell1 S. Beck 2, and G.P. Leonardi, Ph.D.1,3 NASSAU 1 M44 Department of Pathology, Nassau University Medical Center, East Meadow, NY ; UNIVERSITY Department of Biology, Nassau Community College, Garden City, NY2 and Sunrise Medical Laboratories, Hauppauge, NY3 medicalcenter Abstract: Conclusions: Proper specimen collection is the single most important factor in obtaining a reliable and and reweighed. Following addition of water (1.0ml), tubes were shaken at high speeds ß Fiber confi guration and size of the nylon-fl ocked swab allowed for greater accurate diagnostic result. Swabs are often employed to collect specimens for a variety for 1 minute, followed by centrifugation using a table-top Hermle Z180M centrifuge absorbance than the actual nylon material itself. of microbiologic assays. A novel, nylon-tipped, “fl ocked“ swab (Copan, Inc; Corona, CA) (Universal Labnet Co., Edison, NJ) for 5 minutes at maximum speed. Unabsorbed water FIG. 1 FIG. 2 has recently become commercially available. Unlike conventional swabs in which fi bers ß There are numerous other factors, not presently examined, which can aff ect was removed by decanting and wiping the inner tube surface with bibulous paper, swab performance. are woven on a shaft material, fl ocked swabs have a “one piece” construction with nylon and the tube was reweighed. This exercise was repeated twice. fi bers radiating from the shaft tip. This construction may enhance the amount of specimen ß Nevertheless, our data suggests that using of nylon-fl ocked swabs would available for diagnostic testing.