Innovation Academy Symposium Brochure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NACT UK Norfolk House East, 499 Silbury Boulevard, Central Milton Keynes MK9 2AH
NACT UK Norfolk House East, 499 Silbury Boulevard, Central Milton Keynes MK9 2AH Tel: 01908 488033 [email protected] www.nact.org.uk Chair Hon Secretary Hon Treasurer Dr A Cooper Dr R Aspinall Dr A Malin Medical Edn Centre Education Centre Postgraduate Centre Rotherham General University Hospital Bristol Royal United Hospital Hospital NHS Trust NHS Foundation Trust Combe Park Moorgate Road Upper Maudlin Street Bath BA1 3NG Rotherham S60 2UD Bristol BS2 8AE 01225 824891 01709 307868 0117 3420 053 Vice Chairman – Dr S Remington 0161 625 7639 Honorary Assistant Secretary – Dr D Mulherin 01543 576716 Editor “Clinical Tutor” – Dr D McKeon 01248 384621 The National Association of Clinical Tutors (NACT) was originally founded in 1969 to further the interests of what were then called District Clinical Tutors nationally and to help and support them in their work. Our membership has grown since then to encompass the variety of leading educators involved at the local level in the management and delivery of postgraduate medical education across the UK. Through our courses, workshops and conferences, we provide opportunities for our members and others to improve their skills and knowledge in the field of PGME. NACT UK liaises on behalf of its members with many national bodies involved in Medical Education. We communicate our knowledge of these to our membership through a long established information cascade system. To emphasise its role across the UK, on 10th May 2007 the members voted for the organisation to be known as NACT UK. Our association membership is primarily made up of: Clinical Tutors/ Directors of Medical Education/Faculty Leads (Wales), Foundation Programme Directors, SAS Tutors, Training Programme Directors, Associate Deans, College Tutors and MEMs (who are Members of NAMEM) although we are happy to consider anyone involved in PGME who share our aims. -

Discovery of Volatile Biomarkers of Parkinson's Disease from Sebum
bioRxiv preprint doi: https://doi.org/10.1101/469726; this version posted November 15, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Discovery of volatile biomarkers of Parkinson’s disease from sebum 1 sentence summary: Metabolomics identifies volatile odorous compounds from patient sebum that associate with the smell of Parkinson’s. Drupad K Trivedi1, Eleanor Sinclair1, Yun Xu1, Depanjan Sarkar1, Camilla Liscio2, Phine Banks2, Joy Milne1, Monty Silverdale3, Tilo Kunath4, Royston Goodacre1, Perdita Barran*1 1Manchester Institute of Biotechnology, School of Chemistry, The University of Manchester, Princess Street, Manchester, UK, M1 7DN 2Anatune, 4 Wellbrook Way, Girton, Cambridge, UK, CB3 0NA 3Department of Neurology, Salford Royal Foundation Trust, Manchester Academic Health Science Centre, University of Manchester, UK 4Institute for Stem Cell Research, School of Biological Sciences, The University of Edinburgh, Edinburgh UK, EH16 4UU Parkinson’s disease (PD) is a progressive, neurodegenerative disease that presents with significant motor symptoms, for which there is no diagnostic test (1-3). We have serendipitously identified a hyperosmic individual, a ‘Super Smeller’ that can detect PD by odor alone, and our early pilot studies have indicated that the odor was present in the sebum from the skin of PD subjects(4). Here, we have employed an unbiased approach to investigate the volatile metabolites of sebum samples obtained non- invasively from the upper back of 64 participants in total (21 controls and 43 PD subjects). Our results, validated by an independent cohort, identified a distinct volatiles-associated signature of PD, including altered levels of perillic aldehyde and eicosane, the smell of which was then described as being highly similar to the scent of PD by our ‘Super Smeller’. -

Clinical Senior Lecturer JD.Pdf
About Imperial College London Overview Imperial College London is one of the world’s greatest universities, renowned for its ground- breaking research, talented community of staff, students and alumni and its international reach. With a mission to achieve enduring excellence in research and education in science, engineering, medicine and business for the benefit of society, the College was founded in 1907 in South Kensington, bringing together nineteenth century institutions including the Royal College of Science, Royal School of Mines and City and Guilds College. Today Imperial collaborates extensively with neighbouring institutions, including the Royal College of Art and the Royal College of Music. From its location in this great cultural quarter, Imperial provides one of the world’s best educations in STEM subjects for more than 18,400 students, over half of whom come from overseas, reflecting its status as the UK’s most international university. Imperial has three academic faculties – Engineering, Medicine, and Natural Sciences – and the Imperial College Business School, as well as a significant number of interdisciplinary research centres focusing on challenging world problems. The College’s mission is supported by over 8,000 diverse staff, who collaborate in the UK and internationally, often across disciplines. In 2017-2018 the College had a total turnover of over £1 billion, of which £364.2 million directly supported research through grants and contracts. The College’s 2015-2020 Strategy is built on the foundations that make Imperial a strong academic institution and the talented and inspirational people who make up its community. The College’s success is recognised all over the world, as is evidenced by daily coverage of Imperial discoveries and innovations in the international media and claims many distinguished members, including 14 Nobel laureates, three Fields Medallists, and members of the Royal Society and National Academies. -

APPOINTMENT of a CONSULTANT MEDICAL MICROBIOLOGIST Or INFECTIOUS DISEASES and MEDICAL MICROBIOLOGY
APPOINTMENT OF A CONSULTANT MEDICAL MICROBIOLOGIST Or INFECTIOUS DISEASES and MEDICAL MICROBIOLOGY Contents Section Page 1. Job Summary 4 2. Duties and Responsibilities 4-5 - Lead Microbiologist Responsibilities 5 - Infection Prevention and Control 5 - Duties of Infection Control Doctor 6 - Organisational 6 - Clinical Governance, Audit and Quality Management 6 - Management 6-7 - Antimicrobial Stewardship Responsibilities 7 3. Details of the Microbiology Department/Service 7-8 - The microbiology Laboratory 8 4. Proposal/Example of a Weekly Job Plan 9 5. Department Structure – Medical Staffing and Other Staff 9-10 6. Education, Teaching/Training 11 7. Research 11 8. Clinical Governance, Audit and Risk Management 11 9. Induction 11 10. Revalidation & Appraisal 12 11. Raising Concerns 12 12. Secretarial/Administrative Support 12 13. Clinical Excellence Awards 12 14. Private Practice 12 15. Professional Structures 12 16. Preliminary Visits 12 Information about the Trust The Hillingdon Hospitals NHS Foundation Trust and Surrounding Area 13 Shaping a Healthier Future 13 Whole Systems Integration Pilot 13 Trust Cares Values 14 Hospital Management Structure 14 Terms and Conditions of Employment 15 Salary 15 GMC Registration 15 Page Clinical and Professional Responsibilities 15 Confidentiality 15 Job Plan 15 Removal Expenses 16 Medical Clearance 16 Arrangements for Annual/Study Leave 16 Rehabilitation of Offenders Act 1974 16 Disclosure and Barring Service 16 Safeguarding 16 No Smoking Policy 16 Security 16 Health & Safety 17 Infection Prevention -

National Cardiac Arrest Audit Participating Hospitals List England
Updated January 2015 National Cardiac Arrest Audit Participating hospitals list The total number of hospitals signed up to participate in NCAA is 181. England Birmingham and Black Country Non-participant Hereford County Hospital Wye Valley NHS Trust New Cross Hospital The Royal Wolverhampton Hospitals NHS Trust Queen Elizabeth Hospital, Birmingham University Hospital Birmingham NHS Foundation Trust Participant Alexandra Hospital Worcestershire Acute Hospitals NHS Trust Birmingham Heartlands Hospital Heart of England NHS Foundation Trust City Hospital Sandwell and West Birmingham Hospitals NHS Trust Good Hope Hospital Heart of England NHS Foundation Trust Manor Hospital Walsall Healthcare NHS Trust Russells Hall Hospital The Dudley Group of Hospitals NHS Trust Sandwell General Hospital Sandwell and West Birmingham Hospitals NHS Trust Solihull Hospital Heart of England NHS Foundation Trust Worcestershire Royal Hospital Worcestershire Acute Hospitals NHS Trust Central England Non-participant George Eliot Hospital George Eliot Hospital NHS Trust University Hospital Coventry University Hospitals Coventry and Warwickshire NHS Trust Participant Glenfield Hospital University Hospitals of Leicester NHS Trust Kettering General Hospital Kettering General Hospital NHS Foundation Trust Leicester General Hospital University Hospitals of Leicester NHS Trust Leicester Royal Infirmary University Hospitals of Leicester NHS Trust Northampton General Hospital Northampton General Hospital NHS Trust Warwick Hospital South Warwickshire NHS Foundation Trust -

School of Chemistry Focus of Department STEMM Date of Application November 2017 Award Level Silver Institution Athena Date: November 2014 Level: SWAN Award Bronze
Department Application Bronze and Silver Award Name of institution The University of Manchester Department School of Chemistry Focus of department STEMM Date of application November 2017 Award Level Silver Institution Athena Date: November 2014 Level: SWAN award Bronze Contact for application Prof Eric McInnes Must be based in the department Email [email protected] Telephone 0161 275 4469 Departmental website http://www.chemistry.manchester.ac.uk 2 1. LETTER OF ENDORSEMENT FROM THE HEAD OF DEPARTMENT Professor R. E. P. Winpenny School of Chemistry The University of Manchester Oxford Road Manchester M13 9PL +44(0)161 275 4654 [email protected] 27th November 2017 I am delighted to give the full and unequivocal support of the School Management Team and all our staff to this application to renew our Athena SWAN Silver Departmental Award. We have an excellent working group that meets regularly to discuss progress and this has raised awareness of issues. More importantly, we have made real progress in addressing equality and diversity issues since our Silver Award in 2013, and that progress is very clear from the excellent application our SAT team has written. We are strongly committed to equality and diversity because it is the right thing to do: we all want to work in an environment that is as open, fair and friendly as possible. I have been very proud of what the School has achieved in the last few years. We are now in the top five in Europe for research (as measured by the Nature index) and in the top six in the UK for undergraduate education (as measured by the Guardian). -

Discovery Through Innovation Manchester Institute of Biotechnology Discovery Through Innovation Research at the Manchester Institute of Biotechnology
Discovery through Innovation Manchester Institute of Biotechnology Discovery through Innovation Research at the Manchester Institute of Biotechnology Contents Grand Challenge: Biomedical and Healthcare 43 12 Innovation in Action 45 Grand Challenge: Research Centres, Institutes and Facilities Industrial Biotechnology 48 18 Postgraduate and Training 51 Grand Challenge: Science and Society 22 Biofuels and Energy 53 Faculty Honours 57 Selected Publications Spotlight: 26 Centre for Synthetic Biology Office of the Director Nigel Scrutton Director Spotlight: Lesley-Ann Miller Communications Manager Systems Biology Rosalind Le Feuvre Research and Planning Manager 31 Penny Johnson Research Strategy Co-ordinator (EU and Industry) Janet England Support Services Manager Spotlight: Editor Lesley-Ann Miller 34 Text Mining Design & Production WeAreCreation.co.uk Spotlight: www.mib.manchester.ac.uk Technologies [email protected] 37 3 MANCHESTER INSTITUTE OF BIOTECHNOLOGY Driving discovery through innovation Fig. 1.0 Discovery through The Manchester Institute of Biotechnology inform and are informed by our research Grand Challenges and showcasing a Innovation Pipeline (MIB) is one of the leading biotechnology at the molecular, systems and design levels selected portfolio of projects that illustrate research institutes in the world. Focusing on as illustrated in our Discovery through the diversity, quality and dynamism of advanced quantitative approaches to specific Innovation Pipeline (fig 1.0). our research teams. Our reputation as an biotechnology -
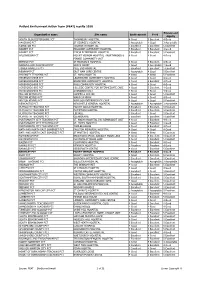
PEAT) Results 2010
Patient Environment Action Team (PEAT) results 2010 Privacy and Organisation name Site name Environment Food dignity SOUTH GLOUCESTERSHIRE PCT THORNBURY HOSPITAL 4 Good 5 Excellent 4 Good HAVERING PCT ST GEORGE'S HOSPITAL 3 Acceptable 4 Good 3 Acceptable KINGSTON PCT TOLWORTH HOSPITAL 5 Excellent 5 Excellent 5 Excellent BARNET PCT EDGWARE COMMUNITY HOSPITAL 5 Excellent 5 Excellent 4 Good BARNET PCT FINCHLEY MEMORIAL HOSPITAL 5 Excellent 5 Excellent 5 Excellent HILLINGDON PCT MOUNT VERNON HOSPITAL - NORTHWOOD & 4 Good 4 Good 4 Good PINNER COMMUNITY UNIT ENFIELD PCT ST MICHAEL'S HOSPITAL 4 Good 5 Excellent 4 Good BARKING AND DAGENHAM PCT GRAYS COURT 4 Good 3 Acceptable 4 Good TOWER HAMLETS PCT MILE END HOSPITAL 5 Excellent 5 Excellent 5 Excellent NEWHAM PCT EAST HAM CARE CENTRE 3 Acceptable 4 Good 5 Excellent HARINGEY TEACHING PCT ST. ANN'S HOSPITAL 4 Good 4 Good 5 Excellent HEREFORDSHIRE PCT LEOMINSTER COMMUNITY HOSPITAL 4 Good 4 Good 4 Good HEREFORDSHIRE PCT BROMYARD COMMUNITY HOSPITAL 4 Good 5 Excellent 4 Good HEREFORDSHIRE PCT ROSS COMMUNITY HOSPITAL 4 Good 4 Good 4 Good HEREFORDSHIRE PCT HILLSIDE CENTRE FOR INTERMEDIATE CARE 4 Good 5 Excellent 4 Good HEREFORDSHIRE PCT STONEBOW UNIT 4 Good 4 Good 4 Good MILTON KEYNES PCT CAMPBELL CENTRE 4 Good 4 Good 5 Excellent MILTON KEYNES PCT WARD 14 MKGH 4 Good 4 Good 4 Good MILTON KEYNES PCT WINDSOR INTERMEDIATE CARE 4 Good 4 Good 5 Excellent NEWCASTLE PCT NEWCASTLE GENERAL HOSPITAL 3 Acceptable 3 Acceptable 3 Acceptable PLYMOUTH TEACHING PCT MOUNT GOULD HOSPITAL 5 Excellent 5 Excellent 5 Excellent PLYMOUTH TEACHING PCT PLYMPTON HOSPITAL 5 Excellent 5 Excellent 5 Excellent PLYMOUTH TEACHING PCT LEE MILL 5 Excellent 4 Good 5 Excellent PLYMOUTH TEACHING PCT GLENBOURNE 5 Excellent 5 Excellent 5 Excellent PORTSMOUTH CITY TEACHING PCT ST. -

North West London Foundation School Prospectus
North West London Foundation School Prospectus Programmes Commencing in August 2020 ______________ North West London Foundation School: Programmes Commencing in August 2020 Introduction Welcome North West London Foundation School, consists of 4 acute Trusts, some of whom have several sites and two mental health Trusts. All have their own distinct personalities, from a teaching hospital with large academic and research departments to district general hospitals in fashionable Chelsea or close to the Heathrow. The commuting distances are relatively small compared to many other Foundation schools, making it easier to live in one location for the whole of the two years. All can be easily reached by public transport. There are 235 standard programmes and 27 academic in total with a wide variety of specialities. They range from neurosurgery at a regional centre to acute medicine in a district general, working with the homeless to young children in community. This prospectus aims to provide you information about your Foundation training in North West London and help you to make choices about where you would like to work. Additional information is on our website: lonkssfoundation.hee.nhs.uk/NWLFS Academic Lead NWLFS Team Dr Channa Jayasena Director Dr Anthea Parry Dr.Channa Jayasena PhD FRCP FRCPath is Academic Director of the LaSE (Imperial) Foundation Programme. He has extensive experience in both postgraduate and undergraduate education, leads his own research laboratory at Imperial, and is a member of the Imperial Clinical Academic Training Board. Dr.Jayasena also works as a clinical lead and Anthea Parry qualified in 1981 from St Bartholomew's consultant in Reproductive Endocrinology & Hospital and has worked as a consultant geriatrician Andrology at Hammersmith Hospital & St. -
Foundation Programme Individual Placement Descriptor* Trust the Hillingdon Hospital NHS Foundation Trust Site the Hillingdon
Foundation Programme Individual Placement Descriptor* Trust The Hillingdon Hospital NHS Foundation Trust Site The Hillingdon Hospital NHS Foundation Trust Trainee Information System (TIS) Post Code (and local post number if known) Placement details (i.e. the specialty F1 Emergency Assessment Unit (EAU) and sub-specialty) Department The Department consists of 4 Consultant Physicians - Dr Edwards, Dr Baburaj, Dr Gerry, Dr Barnes Type of work to expect and Each placement will work towards achieving the learning opportunities competencies in the foundation curriculum. There will be an emphasis on managing the acutely sick patient in a variety of specialties. This will be supplemented by a teaching programme that will augment the F1’s practical experience mapped to the curriculum including topics relating to patient safety. Where the placement is based The Hillingdon Hospital NHS Foundation Trust Clinical supervisor(s) for the Dr Edwards, Dr Baburaj, Dr Gerry, Dr Barnes placement Main duties of the placement In the EAU the job of the F1 is very similar to that of the F2s. The team looks after acute admissions that are only expected to stay for 1-2 days. There is thus a quick turn around of patients. Typical jobs include, discussion of investigation requests with various services, discharge summaries, seeing patients in the EAU review clinic, seeing patients in the EAU HOT clinic, usually with a diagnosis, DVT and other typical ward work. Phlebotomy is generally done by the nursing staff, but there is always the opportunity to practice it. When the EAU team is not busy they are expected to help out the take and see medical patients admitted to hospital with acute medical problems. -

Transformative Healthcare Technologies Engagement Forum 10 & 16 September 2020
Transformative Healthcare Technologies Engagement Forum 10 & 16 September 2020 Attendee List Daniel Abasolo, University of Surrey [email protected] Biomedical Signal Processing, Biomedical Engineering, Non-linear Analysis, Brain, Ageing, Dementia Qammer Abbasi, University of Glasgow [email protected] Remote healthcare technologies, terahertz sensing enabled by machine learning, wearable and implants, non-invasive sensing, 5G and beyond for connected health Abdellatif Abdelgaied, Nottingham Trent University [email protected] Total Joint Replacements, Soft Tissue, Tribology, Biomechanics, Experimental Studies, Computational Simulation Eric Aboagye, Imperial College London [email protected] Cancer, Imaging, positron emission tomography, radiochemistry Andrew Adamatzky, University of the West of England [email protected] Computer modelling, optimisation, drug design, evolutionary computation Tim Adlam, University College London [email protected] Engineering, assistive technology, disability, movement disorders, physical support for children, mobility Prashant Agrawal, Northumbria University [email protected] Fluids dynamics, biomimicry, multiphase flow, blood imbibition Syed Ahmed, University of Manchester [email protected] Industrial Biotechnology, Biocatalysis, Biopharmaceuticals, Food/Beverage and gut microbiome John Ainsworth, University of Manchester [email protected] Digital Health Interventions, Data Science, Systems engineering, Learning Health Systems, -

Your Manchester
your manchesterFor Engineering and Physical Sciences Alumni Spring 2014 Stand up for climate change Stargazing with Leena Gade Tim O’Brien The real value of No1 Engineer & our water ‘man of the year’ THE UNIVERSITY OF MANCHESTER TIMELINE EPS people 1824 Manchester Mechanics’ Institute founded The Faculty is led by the Vice-President and Dean, Professor Colin Bailey, 1851 and comprises nine academic Schools and four Research Institutes. Owens College founded The Faculty Leadership Team also includes six Associate Deans who support key areas of activity, including Research, Teaching and Learning, 1872 Graduate Education, Social Responsibility, Internationalisation and Owens College incorporated the Royal School of Medicine Business Engagement. The Director of Faculty Operations is responsible and Surgery, which had been for leading the administration across the Faculty. formed in 1824 1880 Owens College becomes the School of Chemical Engineering Manchester Institute of Biotechnology first constituent part of the federal and Analytical Science Victoria University, England’s Director, first civic university Head of School, Professor Nigel Scrutton Professor Mike Sutcliffe Photon Science Institute 1883 School of Chemistry Director, The Mechanics’ Institute converted Head of School, Professor Richard Winpenny into the Manchester Technical Professor Christopher Whitehead School and in 1892 becomes University of Manchester Aerospace the Manchester Municipal School of Computer Science Research Institute (UMARI) Technical School Head of School,