Structure and Properties of a Metallocene Polypropylene Resin with Low Melting Temperature for Melt Spinning Fiber Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

EI-ICHI NEGISHI Herbert C
MAGICAL POWER OF TRANSITION METALS: PAST, PRESENT, AND FUTURE Nobel Lecture, December 8, 2010 by EI-ICHI NEGISHI Herbert C. Brown Laboratories of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907-2084, U.S.A. Not long ago, the primary goal of the synthesis of complex natural products and related compounds of biological and medicinal interest was to be able to synthesize them, preferably before anyone else. While this still remains a very important goal, a number of today’s top-notch synthetic chemists must feel and even think that, given ample resources and time, they are capable of synthesizing virtually all natural products and many analogues thereof. Accepting this notion, what would then be the major goals of organic synthesis in the twenty-first century? One thing appears to be unmistakably certain. Namely, we will always need, perhaps increasingly so with time, the uniquely creative field of synthetic organic and organometallic chemistry to prepare both new and existing organic compounds for the benefit and well-being of mankind. It then seems reasonably clear that, in addition to the question of what compounds to synthesize, that of how best to synthesize them will become increasingly important. As some may have said, the primary goal would then shift from aiming to be the first to synthesize a given compound to seeking its ultimately satisfactory or “last synthesis”. If one carefully goes over various aspects of organic synthetic methodology, one would soon note how primitive and limited it had been until rather recently, or perhaps even today. For the sake of argument, we may propose here that the ultimate goal of organic synthesis is “to be able to synthesize any desired and fundamentally synthesizable organic compounds (a) in high yields, (b) efficiently (in as few steps as possible, for example), (c) selectively, preferably all in t98–99% selectivity, (d) economically, and (e) safely, abbreviated hereafter as the y(es)2 manner.” with or without catalyst R1M + R2X R1R2 + MX R1, R2: carbon groups. -

Catalytic Systems Based on Cp2zrx2 (X = Cl, H), Organoaluminum
catalysts Article Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization Lyudmila V. Parfenova 1,* , Pavel V. Kovyazin 1, Almira Kh. Bikmeeva 1 and Eldar R. Palatov 2 1 Institute of Petrochemistry and Catalysis of Russian Academy of Sciences, Prospekt Oktyabrya, 141, 450075 Ufa, Russia; [email protected] (P.V.K.); [email protected] (A.K.B.) 2 Bashkir State University, st. Zaki Validi, 32, 450076 Ufa, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-347-284-3527 i i Abstract: The activity and chemoselectivity of the Cp2ZrCl2-XAlBu 2 (X = H, Bu ) and [Cp2ZrH2]2- ClAlEt2 catalytic systems activated by (Ph3C)[B(C6F5)4] or B(C6F5)3 were studied in reactions with 1-hexene. The activation of the systems by B(C6F5)3 resulted in the selective formation of head- to-tail alkene dimers in up to 93% yields. NMR studies of the reactions of Zr complexes with organoaluminum compounds (OACs) and boron activators showed the formation of Zr,Zr- and Zr,Al-hydride intermediates, for which diffusion coefficients, hydrodynamic radii, and volumes were estimated using the diffusion ordered spectroscopy DOSY. Bis-zirconium hydride clusters of type x[Cp ZrH ·Cp ZrHCl·ClAlR ]·yRnAl(C F ) − were found to be the key intermediates of alkene 2 2 2 2 6 5 3 n dimerization, whereas cationic Zr,Al-hydrides led to the formation of oligomers. Citation: Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R. -

Organometrallic Chemistry
CHE 425: ORGANOMETALLIC CHEMISTRY SOURCE: OPEN ACCESS FROM INTERNET; Striver and Atkins Inorganic Chemistry Lecturer: Prof. O. G. Adeyemi ORGANOMETALLIC CHEMISTRY Definitions: Organometallic compounds are compounds that possess one or more metal-carbon bond. The bond must be “ionic or covalent, localized or delocalized between one or more carbon atoms of an organic group or molecule and a transition, lanthanide, actinide, or main group metal atom.” Organometallic chemistry is often described as a bridge between organic and inorganic chemistry. Organometallic compounds are very important in the chemical industry, as a number of them are used as industrial catalysts and as a route to synthesizing drugs that would not have been possible using purely organic synthetic routes. Coordinative unsaturation is a term used to describe a complex that has one or more open coordination sites where another ligand can be accommodated. Coordinative unsaturation is a very important concept in organotrasition metal chemistry. Hapticity of a ligand is the number of atoms that are directly bonded to the metal centre. Hapticity is denoted with a Greek letter η (eta) and the number of bonds a ligand has with a metal centre is indicated as a superscript, thus η1, η2, η3, ηn for hapticity 1, 2, 3, and n respectively. Bridging ligands are normally preceded by μ, with a subscript to indicate the number of metal centres it bridges, e.g. μ2–CO for a CO that bridges two metal centres. Ambidentate ligands are polydentate ligands that can coordinate to the metal centre through one or more atoms. – – – For example CN can coordinate via C or N; SCN via S or N; NO2 via N or N. -

Cyclopentadienyl Compounds of the First Row Transition Metals And
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2017 Cyclopentadienyl Compounds of the First Row Transition Metals and Early Actinides: Novel Main-Group Bond Forming Catalysis and New Metallacycles Justin Kane Pagano University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Chemistry Commons Recommended Citation Pagano, Justin Kane, "Cyclopentadienyl Compounds of the First Row Transition Metals and Early Actinides: Novel Main-Group Bond Forming Catalysis and New Metallacycles" (2017). Graduate College Dissertations and Theses. 700. https://scholarworks.uvm.edu/graddis/700 This Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. CYCLOPENTADIENYL COMPOUNDS OF THE FIRST ROW TRANSITION METALS AND EARLY ACTINIDES: NOVEL MAIN-GROUP BOND FORMING CATALYSIS AND NEW METALLACYCLES A Dissertation Presented by Justin Kane Pagano to The Faculty of the Graduate College of The University of Vermont In Partial Fulfilment of the Requirements For the Degree of Doctor of Philosophy Specializing in Chemistry May, 2017 Defense Date: November 29, 2016 Dissertation Examination Committee: Rory Waterman, Ph. D., Advisor John M. Hughes, Ph. D., Chairperson Matthias Brewer, Ph. D. Jaqueline L. Kiplinger, Ph. D. Matthew D. Liptak, Ph. D. Cynthia J. Forehand, Ph. D., Dean of the Graduate College ABSTRACT Cyclopentadienyl first row transition-metal compounds have been well studied 5 since the 1950’s, with the nearly ubiquitous CpFe(CO)2Me (FpMe) (Cp = η -C5H5) being one of the first organometallics to be fully characterized. -

Synthesis and Reactivity of Cyclopentadienyl Based Organometallic Compounds and Their Electrochemical and Biological Properties
Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Sasmita Mishra Department of Chemistry National Institute of Technology Rourkela Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties Dissertation submitted to the National Institute of Technology Rourkela In partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry by Sasmita Mishra (Roll Number: 511CY604) Under the supervision of Prof. Saurav Chatterjee February, 2017 Department of Chemistry National Institute of Technology Rourkela Department of Chemistry National Institute of Technology Rourkela Certificate of Examination Roll Number: 511CY604 Name: Sasmita Mishra Title of Dissertation: ''Synthesis and reactivity of cyclopentadienyl based organometallic compounds and their electrochemical and biological properties We the below signed, after checking the dissertation mentioned above and the official record book(s) of the student, hereby state our approval of the dissertation submitted in partial fulfillment of the requirements of the degree of Doctor of Philosophy in Chemistry at National Institute of Technology Rourkela. We are satisfied with the volume, quality, correctness, and originality of the work. --------------------------- Prof. Saurav Chatterjee Principal Supervisor --------------------------- --------------------------- Prof. A. Sahoo. Prof. G. Hota Member (DSC) Member (DSC) --------------------------- -

US20100160506A1.Pdf
US 2010.0160506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0160506 A1 Wu et al. (43) Pub. Date: Jun. 24, 2010 (54) PRODUCTION OFSYNTHETIC Publication Classification HYDROCARBON FLUIDS, PLASTICIZERS (51) Int. Cl. AND SYNTHETICLUBRICANT BASE CSK 5/55 (2006.01) STOCKS FROM RENEWABLE FEEDSTOCKS CSK 5/109 (2006.01) C07D 30/03 (2006.01) (76) Inventors: Margaret May-Som Wu, Skillman, C07D 31 7/24 (2006.01) NJ (US); Karla Schall Colle, C07C I/207 (2006.01) Houston, TX (US); Ramzi Yanni (52) U.S. Cl. ......... 524/114; 524/280; 549/523: 549/229; Saleh, Baton Rouge, LA (US); 585/327 Allen D. Godwin, Seabrook, TX (57) ABSTRACT (US); John Edmond Randolph This disclosure is directed to an integrated method for making Stanat, Houston, TX (US) synthetic hydrocarbon fluids, plasticizers and polar synthetic lubricant base stocks from a renewable feedstock. More par Correspondence Address: ticularly, the disclosure is directed to a metathesis reaction of ExxonMobil Research & Engineering Company natural oil or its derivative ester and ethylene in the presence P.O. Box 900, 1545 Route 22 East of an effective amount of a metathesis catalyst to form linear Annandale, NJ 08801-0900 (US) alpha-olefins, internal olefins and reduced chain length trig lycerides. The linear alpha-olefins and/or internal olefins are polymerized to produce synthetic hydrocarbon fluids in the (21) Appl. No.: 12/633,742 presence of a suitable catalyst. The reduced chain length triglycerides are converted into polar synthetic lubricant base (22) Filed: Dec. 17, 2009 stocks or plasticizers by hydrogenation, isomerization, fol lowed by hydrogenations, or by hydroisomerization pro cesses. -
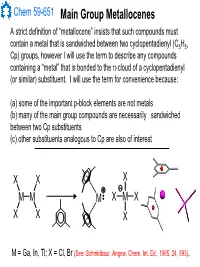
Main Group Metallocenes
Chem 59-651 Main Group Metallocenes A strict definition of “metallocene” insists that such compounds must contain a metal that is sandwiched between two cyclopentadienyl (C5H5, Cp) groups, however I will use the term to describe any compounds containing a “metal” that is bonded to the π-cloud of a cyclopentadienyl (or similar) substituent. I will use the term for convenience because: (a) some of the important p-block elements are not metals (b) many of the main group compounds are necessarily sandwiched between two Cp substituents (c) other substituents analogous to Cp are also of interest XX X MM M XMX X X X M = Ga, In, Tl; X = Cl, Br (See: Schmidbaur, Angew. Chem. Int. Ed., 1985, 24, 893). Chem 59-651 Cyclopentadienyl Ligand Basics The structural features and bonding interactions between cyclopentadienyl groups and metal atoms shows considerable variation and is described using a few different conventions (See Jutzi and Burford, Chem. Rev., 1999, 99, 969 and references therein). In most main group metallocenes, even those that are σ-bonded in the solid state, the rings rotate rapidly through a series of 1,2-shifts. A Cp group can be considered to be p-bonded to an element if the Hapticity: element sits inside the cylinder defined by the 5 carbon atoms of M MM the Cp ligand. The center of mass of the 5 C atoms is known as the centroid. η1-Cp η2-Cp η3-Cp M M M A σ-bonded Cp can be identified η4-Cp η5-Cp ≡η5-Cp by the angle at the ipso carbon M and bond lengths should indicate single bonds to the ipso carbon and a localized diene structure for σ the α and β carbon fragment. -

The Synthesis and Properties of Some Metallocene
THE SYNTHESIS AND PROPERTIES OF SOME METALLOCENE CONTAINING MACROCYCLES AND THEIR ANALOGUES by JONATHAN PAUL KNYCHALA A Thesis presented for the Degree of DOCTOR OF PHILOSOPHY in the Faculty of Science of the UNIVERSITY OF LONDON Bedford College, London ProQuest Number: 10098532 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10098532 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.Q. Box 1346 Ann Arbor, Ml 48106-1346 CONTENTS Page ABSTRACT 7 ACKNOWLEDGEMENTS 9 INTRODUCTION 11 CHAPTER 1 . CROWN-ETHERS AND CRYPTANDS 1 1 1 .1 Introduction 1 1 1 .2 Synthesis 12 1 .3 Structure and selective ion-binding 16 1 .4 The effect of the ligand on the 23 properties of the cation CHAPTER 2. A REVIEW OF THE CURRENT LITERATURE 26 ON THE USES OF CROWN-ETHERS AND CRYPTANDS 2.1 Chemical 26 2.2 Biochemical 47 DISCUSSION CHAPTER 3. DISCUSSION 56 3.1 Aims of the project 56 3.2(i) Structure and physical properties of 58 ferrocene and ruthenocene 3.2(ii) Electrochemistry 61 3.3 The design of ferrocene and ruthenocene 65 macrocycles containing ion-binding ligands Page CHAPTER 4. -

Cyclopentadienyl/Fluorenyl-Group 4 Ansa-Metallocene Catalysts for Production of Tailor-Made Polyolefins Evgueni Kirillov, Jean-François Carpentier
Cyclopentadienyl/Fluorenyl-Group 4 ansa-metallocene Catalysts for Production of Tailor-made Polyolefins Evgueni Kirillov, Jean-François Carpentier To cite this version: Evgueni Kirillov, Jean-François Carpentier. Cyclopentadienyl/Fluorenyl-Group 4 ansa-metallocene Catalysts for Production of Tailor-made Polyolefins. Chemical Record, Chemical Society of Japan, 2021, 21 (2), pp.357-375. 10.1002/tcr.202000142. hal-03102146 HAL Id: hal-03102146 https://hal.archives-ouvertes.fr/hal-03102146 Submitted on 4 Feb 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Manuscript Click here to access/download;Manuscript;Manuscript_tcr.202000142_revis PERSONAL ACCOUNT 1 2 {Cyclopentadienyl/Fluorenyl}-Group 4 ansa-Metallocene Catalysts 3 4 for Production of Tailor-Made Polyolefins 5 6 Evgueni Kirillov*[a] and Jean-François Carpentier*[a] 7 8 9 Dedication: dedicated to the memories of Prof. Malcolm H. Green, deceased July 24, 2020 and Prof. André Mortreux, deceased 10 October 10, 2020. 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 manuscript -

Determination of the Dielectric Constant of Pure and Bamboo Carbonated Polymers Using the Impedance Method
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2015, 6(12): 168-174 ISSN: 0976-8610 CODEN (USA): AASRFC Determination of the Dielectric Constant of Pure and Bamboo Carbonated Polymers using the Impedance Method Anyeji Joy and Okpala U. V. Department of Industrial Physics, Chukwuemeka Odumegwu Ojukwu University, Uli _____________________________________________________________________________________________ ABSTRACT In this research work, we investigated the dielectric constant of pure and carbonated polymers using the impedance method. In carrying out the research, low density polyethylene (LDPE) and metallocene polyethylene (MPE) were used as the polymers. The main difference between LDPE and MPE is that low density polyethylene is produced by high pressure free-radical polymerization of ethylene while MPE is produced by low pressure polymerization technology using metallocene as catalyst to copolymerize ethylene and another monomer such as butane-1. Bamboo charcoal which serves as the carbon was added to each samples. The dielectric constant of the polymers increased with increased weight of carbon. For LDPE, the dielectric constant increased from 2.34 to 7.16 as the percentage of carbon was increased from 0% to 20%. For MPE, the dielectric constant increased from 2.27 to 7.26 as the percentage of carbon was increased from 0% to 20%. This showed that bamboo which is known for its high tensile strength when carbonated with polymers enhanced electrical properties of the polymers. Our result showed that bamboo carbon will also protect the polymer from ultra-violet degradation, being that polyethylene is particularly sensitive to ultra-violet radiation. This showed that bamboo charcoal is good for carbonation of polymer. -

Synthesis, Characterization, and Molecular Structure of Bis(Tetraphenylcyclopentdienyl)Rhodium(II)⊗ James E
Marshall University Marshall Digital Scholar Chemistry Faculty Research Chemistry 1995 Synthesis, Characterization, and Molecular Structure of Bis(tetraphenylcyclopentdienyl)rhodium(II)⊗ James E. Collins Michael Castellani Marshall University, [email protected] Arnold L. Rheingold Edward J. Miller William E. Geiger See next page for additional authors Follow this and additional works at: http://mds.marshall.edu/chemistry_faculty Part of the Chemistry Commons Recommended Citation Collins, J. E.; Castellani, M. P.; Rheingold, A. L.; Miller, E. J.; Geiger, W. E.; Rieger, A. L.; Rieger, P. H., Synthesis, Characterization, and Molecular Structure of Bis (tetraphenylcyclopentdienyl) rhodium (II). Organometallics 1995, 14 (3), 1232-1238. This Article is brought to you for free and open access by the Chemistry at Marshall Digital Scholar. It has been accepted for inclusion in Chemistry Faculty Research by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected], [email protected]. Authors James E. Collins, Michael Castellani, Arnold L. Rheingold, Edward J. Miller, William E. Geiger, Anne L. Rieger, and Philip H. Rieger This article is available at Marshall Digital Scholar: http://mds.marshall.edu/chemistry_faculty/20 Synthesis, Characterization, and Molecular Structure of Bis(tetraphenylcyclopentdienyl)rhodium(II)⊗ James E. Collins,†1Michael P. Castellani,*,† Arnold L. Rheingold,*,‡ Edward J. Miller,§,♦ William E. Geiger,*,§ Anne L. Rieger,∇1and Philip H. Rieger*,∇ Departments of Chemistry, Marshall University, Huntington, West Virginia 25755; University of Delaware, Newark, Delaware, 19716; University of Vermont, Burlington, Vermont, 05405; and Brown University, Providence, Rhode Island, 02912. Abstract A 5 day diglyme reflux of Rh(acac)3 and K(C5HPh4), followed by treatment with aqueous HPF6, produces orange-yellow [(C5HPh4)2Rh]PF6 in 40 - 50% yield. -

Zr-Catalyzed Carbometallation
Zr-Catalyzed Carbometallation C C C C MLn R C C MLn R ML n MLn R C C MLn C C C C MLn Wipf Group Research Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation . Term coined for describing chemical processes involving net addition of carbon-metal bonds to carbon-carbon π-bonds. One of the several patterns for C-C bond formation observable with organotransition-metal complexes along with reductive elimination, migratory insertion, etc. C C C C MLn R C C MLn R ML n MLn R C C MLn C C C C MLn C C MLn C MLn ML C C n Juan Arredondo @ Wipf Group 2 11/14/2004 Timeline . In 1959, Natta first reported the polymerization of propylene with TiCl4/AlEt3. Reaction proceeds with relative asymmetric induction with polymer sections of the same absolute configuration (isotactic). Subsequent work was focused on finding a soluble of group IV metallocene catalyst (Ti, Zr, Hf). Controlled Monocarbometallation of Alkenes and Alkynes . Stereo- and regioselective single-state addition of organometallics species can be a valuable synthetic tool. Normant’s carbocupration (1976) was the first example with high selectivity and reasonable generality. Limitations arose from the thermal instability of the alkylcopper reagents and capricious effects of proximal hetereoatoms. R2 H 1 . 2 R Cu MgX2 + R C CH 2 . R Cu MgX2 . Schwartz’s systematic investigation (1974) of hydrozirconation of alkenes and alkynes with Cp2ZrHCl and observations of its addition to alkenes and alkynes by Wales and coworkers. .Kaminsky’s use of Cp2ZrCl2 and methylaluminoxanes as catalyst for alkene polymerization (1976).