Anderson, Ted L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

As of 02 June 2017 (Updated Regularly) ABOUT MIDEM
HIGHLIGHTS MIDEM 2017 as of 02 June 2017 (updated regularly) ABOUT MIDEM Midem is an annual international b2b event dedicated to the ALEXANDRE DENIOT new music ecosystem, with a tradeshow, conferences, Director of Midem competitions, networking events and live performances. It’s the place where music makers, cutting-edge technologies, brands & talents come together to enrich the passionate relationship Alexandre Deniot has been named between people & music, transform audience engagement and Director of Midem in January 2017. form new business connections. He brings with him over 15 years of experience within the music sector, mainly at Universal Music Group where he was most recently Business Development Director for UMG’s Paris-based digital division. In this capacity over the past two years, he spearheaded the development of Universal Music’s digital services in emerging markets and more specifically in Africa. Alexandre Deniot started his career in the music business in 2001 as part of Sony Music Entertainment’s sales The international music ecosystem comes to Midem: marketing team in Lyon, France. He joined Universal Music Group in 2002 as Regional Sales Manager for eastern France then southern France before being promoted to Key Account Manager (Physical Sales) in 2006 and Key Account Manager (Digital Sales) in 2009. Five years later he was named Head of Business Development at Universal Music Group’s digital division, managing Universal Music On Line, a specialist digital subsidiary of Universal Music. In January 2016 he took on the Business Development Director within the digital division in Paris. 2 MIDEM STORY WHERE BUSINESS HAPPENS WHERE TALENT SHINES WHERE IDEAS GROW As the essential relationship broker of the Discover new talent thanks to leading Get inspired by industry leaders from around international music ecosystem, Midem helps industry competitions: the world who share their in sight on today you grow your business. -

Thomas Logging Drive for Success Page 14
issue 45 | march 2017 OFFICIAL PUBLICATION OF TIGERCAT INDUSTRIES INC. Thomas Logging Drive for Success Page 14 New Machines 2 Purpose-built for Longevity 18 A Symbiotic Relationship 7 Letters 21 TFA Diesel Fuel Testing 11 Event Round-up 22 Forestry Tire Service Tips 12 Employee News 24 170511 btb.indd 1 2017-03-29 8:55 AM product news NEW 480B AND TIGERCAT 4061 MULCHING HEAD The robust 480B mulcher equipped with the new Tigercat 4061 mulching head. Tigercat has introduced the powerful, 480B The effi cient, responsive closed loop track drive system delivers powerful tractive effort and performance with mulcher and the brand new Tigercat 4061 greater hydraulic effi ciency in challenging terrain. The mulching head. high-lift boom geometry means high productivity in The Tigercat 480B tackles the toughest terrain with slopes and gullies. ease and offers superior durability and high uptime The cab interior is quiet and instrumentation has been for large-scale, time-sensitive right-of-way and site placed to optimize productivity. The electronic control preparation projects. system interface uses a high-resolution, 180 mm (7 in) With a power rating of 411 kW (550 hp) at 1,800 rpm, touch screen. The 480B is equipped with a comfortable, the Tigercat FPT C13 Tier 4f engine offers quick load heated and cooled seat with air-ride suspension. response, low operating costs and high power density, Excellent cab insulation translates to reduced noise combined with excellent fuel economy. Best of all, levels so operators can take full advantage of the Tigercat FPT engines are backed by Tigercat warranty Bluetooth® sound system with hands-free calling. -
2020: the Biggest Island News of the Year
Inside the Moon Island Holiday A2 On the Rocks A11 Snowy Plover A14 Three Chords and the Truth A16 Issue 872 The 27° 37' 0.5952'' N | 97° 13' 21.4068'' W Island Free The voiceMoon of The Island since 1996 December 31, 2020 Weekly www.islandmoon.com FREE Photo by Debbie Noble Around The Flour Bluff Island 2020: The Biggest Island Plays Veterans By Dale Rankin What a year it has been Around The Memorial in Island. It has been a year of change and it seems 2020 in retrospect News of the Year Fourth Round of will be a tipping point in Island development. As things go our little Playoffs sandbar has fared better than many Water Exchange Bridge Island Grocery Store parts of the country as the Covid virus January 2 brought new visitors to our shores and Canal Set to Open many of whom bought property and decided to stay. Buc Stadium As 2020 winds down realtors, showdown contractors, mortgage lenders, and The Hornet Football Team beat appraisers report booming sales Victoria West 28-21 in Alamo with a lack of inventory becoming Stadium to advance to the Fourth a problem not only on Padre Island Round of the UIL State Playoffs. The but in Port Aransas and across the Hornets will play the Corpus Christi city. Condominiums have been in Veterans Memorial Eagles in the 5A big demand as well as empty dry Division 1 Regional Final Playoff lots which are now regularly selling on Saturday, January 2, 2:00 p.m. at in the $80,000 and up range. -

The Top 7000+ Pop Songs of All-Time 1900-2017
The Top 7000+ Pop Songs of All-Time 1900-2017 Researched, compiled, and calculated by Lance Mangham Contents • Sources • The Top 100 of All-Time • The Top 100 of Each Year (2017-1956) • The Top 50 of 1955 • The Top 40 of 1954 • The Top 20 of Each Year (1953-1930) • The Top 10 of Each Year (1929-1900) SOURCES FOR YEARLY RANKINGS iHeart Radio Top 50 2018 AT 40 (Vince revision) 1989-1970 Billboard AC 2018 Record World/Music Vendor Billboard Adult Pop Songs 2018 (Barry Kowal) 1981-1955 AT 40 (Barry Kowal) 2018-2009 WABC 1981-1961 Hits 1 2018-2017 Randy Price (Billboard/Cashbox) 1979-1970 Billboard Pop Songs 2018-2008 Ranking the 70s 1979-1970 Billboard Radio Songs 2018-2006 Record World 1979-1970 Mediabase Hot AC 2018-2006 Billboard Top 40 (Barry Kowal) 1969-1955 Mediabase AC 2018-2006 Ranking the 60s 1969-1960 Pop Radio Top 20 HAC 2018-2005 Great American Songbook 1969-1968, Mediabase Top 40 2018-2000 1961-1940 American Top 40 2018-1998 The Elvis Era 1963-1956 Rock On The Net 2018-1980 Gilbert & Theroux 1963-1956 Pop Radio Top 20 2018-1941 Hit Parade 1955-1954 Mediabase Powerplay 2017-2016 Billboard Disc Jockey 1953-1950, Apple Top Selling Songs 2017-2016 1948-1947 Mediabase Big Picture 2017-2015 Billboard Jukebox 1953-1949 Radio & Records (Barry Kowal) 2008-1974 Billboard Sales 1953-1946 TSort 2008-1900 Cashbox (Barry Kowal) 1953-1945 Radio & Records CHR/T40/Pop 2007-2001, Hit Parade (Barry Kowal) 1953-1935 1995-1974 Billboard Disc Jockey (BK) 1949, Radio & Records Hot AC 2005-1996 1946-1945 Radio & Records AC 2005-1996 Billboard Jukebox -

1St Edition Brochure
Anyone doing a job they enjoy is lucky. I’m super-double-lucky to not only survive and thrive as a musician but as a musician doing what they dreamt of.. Many and varied live shows to wonderful appreciative audiences, (yes, you guys!) with fantastic players. Sigh. Couldn’t wish for more. So many musicians don’t end up doing what they really want. So many thanks y’all. Keep spreading the word.. please.. Snake The Snake Davis Trio album is out now. We’ll be bringing it on the road with us (cash only please). It’s available directly through our website www.snakedavis.rocks or www.johnnythirkell.com or by post. Cheques payable to Skin Records Ltd £12 each includes p&p to the UK. Address as below. Please include your return address. If you’re outside the UK, drop us a note for postage costs. Please Note :- Some performances might not be on sale yet. Further dates constantly being added. We’ve listed as many performances as we can, to allow all of the busy people out there, a chance to plan ahead. All information is correct to the best of our knowledge but is subject to change so please check with venues prior to booking. We aim to keep the website www.snakedavis.rocks as up to date as possible too. It’s always a good place to check to see if additional dates or details have been added. Our contact details:-email [email protected] www.snakedavis.rocks Snake Davis Skin Records Ltd, 53 High Street, Wootton, Ulceby, DN39 6RP HELMSLEY - Friday 24th January The Snake Davis Band with the 4 piece line up; Snake on his various instruments and a great selection of tunes. -

Team Piraeus: an Exciting Journey Ahead Forward Thinking: the Future of Shipping Contents Leader Another Virtual AGM
No .2 | 2021 149th AGM SPECIAL Team Piraeus: An exciting journey ahead Forward Thinking: The future of shipping Contents Leader Another virtual AGM ..............................................................................................................3 AGM2021 The Swedish Club’s 149th AGM ............................................................................................4 Are you brainfit? ......................................................................................................................8 Interview: Anders Hansen, Guest Speaker ..........................................................................12 New Board members ............................................................................................................13 P8 Forward Thinking Charting the course to automation ......................................................................................14 Norway’s Green Shipping Programme ................................................................................19 Preparing for the extraordinary - the Alfa Lift ....................................................................22 Loss Prevention Safety scenario: Swept away by large wave ......................................................................26 P19 P&I Going home - repatriating seafarers with mental health issues ......................................28 Legal Important clarification of COLREGS ....................................................................................32 Team Piraeus P28 An exciting journey ahead: -

TOP HITS of the EIGHTIES by YEAR 1980’S Top 100 Tracks Page 2 of 31
1980’S Top 100 Tracks Page 1 of 31 TOP HITS OF THE EIGHTIES BY YEAR 1980’S Top 100 Tracks Page 2 of 31 1980 01 Frankie Goes To Hollywood Relax 02 Frankie Goes To Hollywood Two Tribes 03 Stevie Wonder I Just Called To Say I Love You 04 Black Box Ride On Time 05 Jennifer Rush The Power Of Love 06 Band Aid Do They Know It's Christmas? 07 Culture Club Karma Chameleon 08 Jive Bunny & The Mastermixers Swing The Mood 09 Rick Astley Never Gonna Give You Up 10 Ray Parker Jr Ghostbusters 11 Lionel Richie Hello 12 George Michael Careless Whisper 13 Kylie Minogue I Should Be So Lucky 14 Starship Nothing's Gonna Stop Us Now 15 Elaine Paige & Barbara Dickson I Know Him So Well 16 Kylie & Jason Especially For You 17 Dexy's Midnight Runners Come On Eileen 18 Billy Joel Uptown Girl 19 Sister Sledge Frankie 20 Survivor Eye Of The Tiger 21 T'Pau China In Your Hand 22 Yazz & The Plastic Population The Only Way Is Up 23 Soft Cell Tainted Love 24 The Human League Don't You Want Me 25 The Communards Don't Leave Me This Way 26 Jackie Wilson Reet Petite 27 Paul Hardcastle 19 28 Irene Cara Fame 29 Berlin Take My Breath Away 30 Madonna Into The Groove 31 The Bangles Eternal Flame 32 Renee & Renato Save Your Love 33 Black Lace Agadoo 34 Chaka Khan I Feel For You 35 Diana Ross Chain Reaction 36 Shakin' Stevens This Ole House 37 Adam & The Ants Stand And Deliver 38 UB40 Red Red Wine 1980’S Top 100 Tracks Page 3 of 31 39 Boris Gardiner I Want To Wake Up With You 40 Soul II Soul Back To Life 41 Whitney Houston Saving All My Love For You 42 Billy Ocean When The Going -

Cutting Crew Broadcast Mp3, Flac, Wma
Cutting Crew Broadcast mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Electronic / Rock / Pop Album: Broadcast Country: UK Released: 1986 Style: New Wave, Pop Rock, Synth-pop MP3 version RAR size: 1978 mb FLAC version RAR size: 1997 mb WMA version RAR size: 1131 mb Rating: 4.9 Votes: 664 Other Formats: MP3 MP2 VOX DMF VQF AHX AC3 Tracklist Hide Credits Any Colour A1 4:41 Sequenced By – Peter Vitesse*Written-By – MacMichael*, Eede* One For The Mocking-bird A2 4:20 Keyboards – Peter Vitesse*Sequenced By – Pete AdamsWritten-By – Eede* I've Been In Love Before A3 5:18 Keyboards – David Le Boult*Percussion – Jimmy MaelenWritten-By – Eede* Life In A Dangerous Time A4 4:30 Bass – Chris TownsendKeyboards – Peter Vitesse*Written-By – Eede* Fear Of Falling A5 Featuring – The Elephant & Castle Yob ChoirKeyboards – Peter Vitesse*Written-By – 4:52 MacMichael*, Eede* (I Just) Died In Your Arms B1 4:37 Backing Vocals – Pete Birch*Keyboards – Peter Vitesse*Written-By – Eede* Don't Look Back B2 4:08 Keyboards – Peter Vitesse*Saxophone – Gary BarnacleWritten-By – MacMichael*, Eede* Sahara B3 4:53 Keyboards – Peter Vitesse*Saxophone – Gary BarnacleWritten-By – Eede*, Boorer* It Shouldn't Take Too Long B4 Computer [Fairlight] – Peter Woodroffe*Keyboards – Peter Vitesse*Sequenced By – Pete 4:06 AdamsWritten-By – Farley*, MacMichael*, Norman*, Beedle*, Eede* The Broadcast B5 Computer [Fairlight] – Peter Woodroffe*Keyboards – Peter Vitesse*Written-By – Chris 6:38 Townsend, Eede* Companies, etc. Phonographic Copyright (p) – Siren Records Ltd. Copyright (c) -

Industrial Profile
FLEXIBLE AND ADAPTIVE ABOUT METRONOR COMPONENT TECHNOLOGY Metronor is a high technology company headquartered just outside Oslo, Norway, with subsidiaries in the US, Germany and China, globally supporting partners and customers. Based on in-house innovation and research, The concept of Metronor technology relies on Metronor has since 1989 developed a range of two things – a camera and a handheld Lightpen. electro-optical portable coordinate measuring One or more cameras observe a known pattern of systems that have become popular among leading light sources on the Lightpen, and determine the manufacturers worldwide. position and orientation of the pattern in space – in all 6 degrees of freedom. This component approach makes it easy to adapt our systems to your measurement needs. Metronor’s management system is certified to ISO Cameras can be combined to allow for a larger 9001 and complies with ISO 14001. field of view or higher accuracy, and Lightpen models can be interchanged for a wider range of measurement distances. A truly flexible and portable solution, so that all your needs can be covered with a single system that is easy to use. For more information, please visit our website Flexible and robust, the www.metronor.com nature of the Metronor systems enables them to be applied to a Presenting multitude of different Metronor Offices applications. The systems Norway (HQ) | Phone: +47 66 98 38 00 | email: [email protected] are considered especially Germany | Phone: +49 6806 994 0640 | email: [email protected] Industrial Profile China | Phone: +86 10 6447 3936 | email: [email protected] valuable when used USA | Phone: +1 815 381 0920 | email: [email protected] www.metronor.com for large measurement volumes, to optimize processes and in challenging environments. -

List by Song Title
Song Title Artists 4 AM Goapele 73 Jennifer Hanson 1969 Keith Stegall 1985 Bowling For Soup 1999 Prince 3121 Prince 6-8-12 Brian McKnight #1 *** Nelly #1 Dee Jay Goody Goody #1 Fun Frankie J (Anesthesia) - Pulling Teeth Metallica (Another Song) All Over Again Justin Timberlake (At) The End (Of The Rainbow) Earl Grant (Baby) Hully Gully Olympics (The) (Da Le) Taleo Santana (Da Le) Yaleo Santana (Do The) Push And Pull, Part 1 Rufus Thomas (Don't Fear) The Reaper Blue Oyster Cult (Every Time I Turn Around) Back In Love Again L.T.D. (Everything I Do) I Do It For You Brandy (Get Your Kicks On) Route 66 Nat King Cole (Ghost) Riders In The Sky Johnny Cash (Goin') Wild For You Baby Bonnie Raitt (Hey Won't You Play) Another Somebody Done Somebody WrongB.J. Thomas Song (I Can't Get No) Satisfaction Otis Redding (I Don't Know Why) But I Do Clarence "Frogman" Henry (I Got) A Good 'Un John Lee Hooker (I Hate) Everything About You Three Days Grace (I Just Want It) To Be Over Keyshia Cole (I Just) Died In Your Arms Cutting Crew (The) (I Just) Died In Your Arms Cutting Crew (The) (I Know) I'm Losing You Temptations (The) (I Love You) For Sentimental Reasons Nat King Cole (I Love You) For Sentimental Reasons Nat King Cole (I Love You) For Sentimental Reasons ** Sam Cooke (If Only Life Could Be Like) Hollywood Killian Wells (If You're Not In It For Love) I'm Outta Here Shania Twain (If You're Not In It For Love) I'm Outta Here! ** Shania Twain (I'm A) Stand By My Woman Man Ronnie Milsap (I'm Not Your) Steppin' Stone Monkeys (The) (I'm Settin') Fancy Free Oak Ridge Boys (The) (It Must Have Been Ol') Santa Claus Harry Connick, Jr. -

Safety and Shipping Review 2017
Allianz Global Corporate & Specialty Safety and Shipping Review 2017 An annual review of trends and developments in shipping losses and safety Allianz Global Corporate and Specialty The Ocean Dream cruise ship had been abandoned by its owner for over a year before it sank. Photo: ShipSpotting.com. The Modern Express was one of the largest total losses recorded during 2016. CAN S 1 was one of the total losses in the East Mediterranean in 2016 – the top incident hotspot. Photo: iStock Photo: ShipSpotting.com Executive Summary International shipping transports approximately 90% of driven by bad weather. The number of losses resulting world tradei, so the safety of vessels is critical to the global from fire/explosion (8) is up slightly year-on-year. economy. The maritime industry saw the number of total shipping losses decline during 2016 to 85. The number of There were 2,611 reported shipping casualties during shipping incidents (casualties) also declined year-on-year. 2016, down 4%. Machinery damage/engine failure is the main cause and was also responsible for driving a 16% Shipping losses declined by 16% compared with a year increase in the top hotspot – the East Mediterranean & earlier (101). The preliminary figures for the accident Black Sea region. year show a significant improvement on the 10-year loss average (119) – down 29%. Large shipping losses have Growing complexity and interconnectivity of also declined by 50% over the past decade, driven by shipping risk: While the decline in the number of total improved regulation and the development of a more losses and casualties is encouraging, there is no room for robust safety culture. -
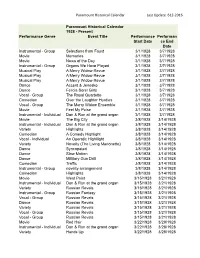
Web Paramount Historical Calendar 6-12-2016.Xlsx
Paramount Historical Calendar Last Update 612-2016 Paramount Historical Calendar 1928 - Present Performance Genre Event Title Performance Performan Start Date ce End Date Instrumental - Group Selections from Faust 3/1/1928 3/7/1928 Movie Memories 3/1/1928 3/7/1928 Movie News of the Day 3/1/1928 3/7/1928 Instrumental - Group Organs We Have Played 3/1/1928 3/7/1928 Musical Play A Merry Widow Revue 3/1/1928 3/7/1928 Musical Play A Merry Widow Revue 3/1/1928 3/7/1928 Musical Play A Merry Widow Revue 3/1/1928 3/7/1928 Dance Accent & Jenesko 3/1/1928 3/7/1928 Dance Felicia Sorel Girls 3/1/1928 3/7/1928 Vocal - Group The Royal Quartette 3/1/1928 3/7/1928 Comedian Over the Laughter Hurdles 3/1/1928 3/7/1928 Vocal - Group The Merry Widow Ensemble 3/1/1928 3/7/1928 Movie Feel My Pulse 3/1/1928 3/7/1928 Instrumental - Individual Don & Ron at the grand organ 3/1/1928 3/7/1928 Movie The Big City 3/8/1928 3/14/1928 Instrumental - Individual Don & Ron at the grand organ 3/8/1928 3/14/1928 Variety Highlights 3/8/1928 3/14/1928 Comedian A Comedy Highlight 3/8/1928 3/14/1928 Vocal - Individual An Operatic Highllight 3/8/1928 3/14/1928 Variety Novelty (The Living Marionette) 3/8/1928 3/14/1928 Dance Syncopated 3/8/1928 3/14/1928 Dance Slow Motion 3/8/1928 3/14/1928 Dance Millitary Gun Drill 3/8/1928 3/14/1928 Comedian Traffic 3/8/1928 3/14/1928 Instrumental - Group novelty arrangement 3/8/1928 3/14/1928 Comedian Highlights 3/8/1928 3/14/1928 Movie West Point 3/15/1928 3/21/1928 Instrumental - Individual Don & Ron at the grand organ 3/15/1928 3/21/1928 Variety