Suppl. Tab. 1. Best BLAST Hit for Each Viral Contig Identified in Leech and Waterhole Samples
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bovine Ephemeral Fever in Asia: Recent Status and Research Gaps
viruses Review Bovine Ephemeral Fever in Asia: Recent Status and Research Gaps Fan Lee Epidemiology Division, Animal Health Research Institute; New Taipei City 25158, Taiwan, China; [email protected]; Tel.: +886-2-26212111 Received: 26 March 2019; Accepted: 2 May 2019; Published: 3 May 2019 Abstract: Bovine ephemeral fever is an arthropod-borne viral disease affecting mainly domestic cattle and water buffalo. The etiological agent of this disease is bovine ephemeral fever virus, a member of the genus Ephemerovirus within the family Rhabdoviridae. Bovine ephemeral fever causes economic losses by a sudden drop in milk production in dairy cattle and loss of condition in beef cattle. Although mortality resulting from this disease is usually lower than 1%, it can reach 20% or even higher. Bovine ephemeral fever is distributed across many countries in Asia, Australia, the Middle East, and Africa. Prevention and control of the disease mainly relies on regular vaccination. The impact of bovine ephemeral fever on the cattle industry may be underestimated, and the introduction of bovine ephemeral fever into European countries is possible, similar to the spread of bluetongue virus and Schmallenberg virus. Research on bovine ephemeral fever remains limited and priority of investigation should be given to defining the biological vectors of this disease and identifying virulence determinants. Keywords: Bovine ephemeral fever; Culicoides biting midge; mosquito 1. Introduction Bovine ephemeral fever (BEF), also known as three-day sickness or three-day fever [1], is an arthropod-borne viral disease that mainly strikes cattle and water buffalo. This disease was first recorded in the late 19th century. -

MALE GENITAL ORGANS and ACCESSORY GLANDS of the LESSER MOUSE DEER, TRAGULUS Fa VAN/CUS
MALE GENITAL ORGANS AND ACCESSORY GLANDS OF THE LESSER MOUSE DEER, TRAGULUS fA VAN/CUS M. K. VIDYADARAN, R. S. K. SHARMA, S. SUMITA, I. ZULKIFLI, AND A. RAZEEM-MAZLAN Faculty of Biomedical and Health Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia (MKV), Faculty of Veterinary Medicine and Animal Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia (RSKS, SS, /Z), Downloaded from https://academic.oup.com/jmammal/article/80/1/199/844673 by guest on 01 October 2021 Department of Wildlife and National Parks, Zoo Melaka, 75450 Melaka, Malaysia (ARM) Gross anatomical features of the male genital organs and accessory genital glands of the lesser mouse deer (Tragulus javanicus) are described. The long fibroelastic penis lacks a prominent glans and is coiled at its free end to form two and one-half turns. Near the tight coils of the penis, on the right ventrolateral aspect, lies a V-shaped ventral process. The scrotum is prominent, unpigmented, and devoid of hair and is attached close to the body, high in the perineal region. The ovoid, obliquely oriented testes carry a large cauda and caput epididymis. Accessory genital glands consist of paired, lobulated, club-shaped vesic ular glands, and a pair of ovoid bulbourethral glands. A well-defined prostate gland was not observed on the surface of the pelvic urethra. Many features of the male genital organs of T. javanicus are pleisomorphic, being retained from suiod ancestors of the Artiodactyla. Key words: Tragulus javanicus, male genital organs, accessory genital glands, reproduc tion, anatomy, Malaysia The lesser mouse deer (Tragulus javan gulidae, and Bovidae (Webb and Taylor, icus), although a ruminant, possesses cer 1980). -

A Scoping Review of Viral Diseases in African Ungulates
veterinary sciences Review A Scoping Review of Viral Diseases in African Ungulates Hendrik Swanepoel 1,2, Jan Crafford 1 and Melvyn Quan 1,* 1 Vectors and Vector-Borne Diseases Research Programme, Department of Veterinary Tropical Disease, Faculty of Veterinary Science, University of Pretoria, Pretoria 0110, South Africa; [email protected] (H.S.); [email protected] (J.C.) 2 Department of Biomedical Sciences, Institute of Tropical Medicine, 2000 Antwerp, Belgium * Correspondence: [email protected]; Tel.: +27-12-529-8142 Abstract: (1) Background: Viral diseases are important as they can cause significant clinical disease in both wild and domestic animals, as well as in humans. They also make up a large proportion of emerging infectious diseases. (2) Methods: A scoping review of peer-reviewed publications was performed and based on the guidelines set out in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews. (3) Results: The final set of publications consisted of 145 publications. Thirty-two viruses were identified in the publications and 50 African ungulates were reported/diagnosed with viral infections. Eighteen countries had viruses diagnosed in wild ungulates reported in the literature. (4) Conclusions: A comprehensive review identified several areas where little information was available and recommendations were made. It is recommended that governments and research institutions offer more funding to investigate and report viral diseases of greater clinical and zoonotic significance. A further recommendation is for appropriate One Health approaches to be adopted for investigating, controlling, managing and preventing diseases. Diseases which may threaten the conservation of certain wildlife species also require focused attention. -

And Filoviruses Asit K
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Veterinary and Biomedical Science Veterinary and Biomedical Sciences, Department of 2016 Overview of Rhabdo- and Filoviruses Asit K. Pattnaik University of Nebraska-Lincoln, [email protected] Michael A. Whitt University of Tennessee Health Science Center, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/vetscipapers Part of the Biochemistry, Biophysics, and Structural Biology Commons, Cell and Developmental Biology Commons, Immunology and Infectious Disease Commons, Medical Sciences Commons, Veterinary Microbiology and Immunobiology Commons, and the Veterinary Pathology and Pathobiology Commons Pattnaik, Asit K. and Whitt, Michael A., "Overview of Rhabdo- and Filoviruses" (2016). Papers in Veterinary and Biomedical Science. 229. http://digitalcommons.unl.edu/vetscipapers/229 This Article is brought to you for free and open access by the Veterinary and Biomedical Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Veterinary and Biomedical Science by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Biology and Pathogenesis of Rhabdo- and Filoviruses (2016), edited by Asit K Pattnaik and Michael A Whitt. Copyright © 2016 World Scientific Publishing Co Pte Ltd. Used by permission. digitalcommons.unl.edu CHAPTER 1 Overview of Rhabdo- and Filoviruses Asit K. Pattnaik1 and Michael A. Whitt2 1 School of Veterinary Medicine and Biomedical Sciences and Nebraska Center for Virology, University of Nebraska-Lincoln, Lincoln, Nebraska 68583 2 Department of Microbiology, Immunology, and Biochemistry, University of Tennessee Health Science Center, Memphis, Tennessee 38163 The authors contributed equally to this work. Emails: [email protected] ; [email protected] Summary Enveloped viruses with a negative-sense, single-stranded monopartite RNA genome have been classified into the orderMononegavirales . -

Cervid Mixed-Species Table That Was Included in the 2014 Cervid RC
Appendix III. Cervid Mixed Species Attempts (Successful) Species Birds Ungulates Small Mammals Alces alces Trumpeter Swans Moose Axis axis Saurus Crane, Stanley Crane, Turkey, Sandhill Crane Sambar, Nilgai, Mouflon, Indian Rhino, Przewalski Horse, Sable, Gemsbok, Addax, Fallow Deer, Waterbuck, Persian Spotted Deer Goitered Gazelle, Reeves Muntjac, Blackbuck, Whitetailed deer Axis calamianensis Pronghorn, Bighorned Sheep Calamian Deer Axis kuhili Kuhl’s or Bawean Deer Axis porcinus Saurus Crane Sika, Sambar, Pere David's Deer, Wisent, Waterbuffalo, Muntjac Hog Deer Capreolus capreolus Western Roe Deer Cervus albirostris Urial, Markhor, Fallow Deer, MacNeil's Deer, Barbary Deer, Bactrian Wapiti, Wisent, Banteng, Sambar, Pere White-lipped Deer David's Deer, Sika Cervus alfredi Philipine Spotted Deer Cervus duvauceli Saurus Crane Mouflon, Goitered Gazelle, Axis Deer, Indian Rhino, Indian Muntjac, Sika, Nilgai, Sambar Barasingha Cervus elaphus Turkey, Roadrunner Sand Gazelle, Fallow Deer, White-lipped Deer, Axis Deer, Sika, Scimitar-horned Oryx, Addra Gazelle, Ankole, Red Deer or Elk Dromedary Camel, Bison, Pronghorn, Giraffe, Grant's Zebra, Wildebeest, Addax, Blesbok, Bontebok Cervus eldii Urial, Markhor, Sambar, Sika, Wisent, Waterbuffalo Burmese Brow-antlered Deer Cervus nippon Saurus Crane, Pheasant Mouflon, Urial, Markhor, Hog Deer, Sambar, Barasingha, Nilgai, Wisent, Pere David's Deer Sika 52 Cervus unicolor Mouflon, Urial, Markhor, Barasingha, Nilgai, Rusa, Sika, Indian Rhino Sambar Dama dama Rhea Llama, Tapirs European Fallow Deer -

ICTV Code Assigned: 2011.001Ag Officers)
This form should be used for all taxonomic proposals. Please complete all those modules that are applicable (and then delete the unwanted sections). For guidance, see the notes written in blue and the separate document “Help with completing a taxonomic proposal” Please try to keep related proposals within a single document; you can copy the modules to create more than one genus within a new family, for example. MODULE 1: TITLE, AUTHORS, etc (to be completed by ICTV Code assigned: 2011.001aG officers) Short title: Change existing virus species names to non-Latinized binomials (e.g. 6 new species in the genus Zetavirus) Modules attached 1 2 3 4 5 (modules 1 and 9 are required) 6 7 8 9 Author(s) with e-mail address(es) of the proposer: Van Regenmortel Marc, [email protected] Burke Donald, [email protected] Calisher Charles, [email protected] Dietzgen Ralf, [email protected] Fauquet Claude, [email protected] Ghabrial Said, [email protected] Jahrling Peter, [email protected] Johnson Karl, [email protected] Holbrook Michael, [email protected] Horzinek Marian, [email protected] Keil Guenther, [email protected] Kuhn Jens, [email protected] Mahy Brian, [email protected] Martelli Giovanni, [email protected] Pringle Craig, [email protected] Rybicki Ed, [email protected] Skern Tim, [email protected] Tesh Robert, [email protected] Wahl-Jensen Victoria, [email protected] Walker Peter, [email protected] Weaver Scott, [email protected] List the ICTV study group(s) that have seen this proposal: A list of study groups and contacts is provided at http://www.ictvonline.org/subcommittees.asp . -

2013-Ross Et Al
JOBNAME: No Job Name PAGE: 1 SESS: 9 OUTPUT: Thu Jan 31 01:23:45 2013 /v2451/blackwell/3G_journals/jzo_v0_i0/jzo_12018 Toppan Best-set Premedia Limited Journal Code: JZO Proofreader: Mony Article No: JZO12018 Delivery date: 30 Jan 2013 Page Extent: 11 Journal of Zoology. Print ISSN 0952-8369 Activity patterns and temporal avoidance by prey in response to Sunda clouded leopard predation risk J. Ross1*,2, A. J. Hearn1*,2 P. J. Johnson1 & D. W. Macdonald1 1 Wildlife Conservation Research Unit (WildCRU), Department of Zoology, University of Oxford, Oxford, UK 2 2 Global Canopy Programme, Oxford, UK bs_bs_query Keywords Abstract activity patterns; circular statistics; overlap coefficient; Sunda clouded leopard; Little is known about the activity patterns of Bornean ungulates, or the temporal ungulate. interactions of these species with the Sunda clouded leopard Neofelis diardi. In this study, we use photographic capture data to quantify the activity patterns for the Correspondence Sunda clouded leopard and six potential prey species: bearded pig Sus barbatus, Joanna Ross, Wildlife Conservation Bornean yellow muntjac Muntiacus atherodes, red muntjac Muntiacus muntjak, Research Unit, Department of Zoology, lesser mouse deer Tragulus kanchil, greater mouse deer Tragulus napu, and sambar University of Oxford, The Recanati-Kaplan deer Rusa unicolor, and to calculate the overlap in activity patterns between these Centre, Tubney House, Abingdon Road, species. This is the first insight into the temporal interactions between the Sunda Tubney, Abingdon OX13 5QL, UK. clouded leopard and its potential prey. Sunda clouded leopards’ activity patterns Email: [email protected] overlapped most with those of sambar deer and greater mouse deer. -

The (Sleeping) Beauty in the Beast T1 Extendash a Review on the Water
Published by Associazione Teriologica Italiana Volume 28 (2): 121–133, 2017 Hystrix, the Italian Journal of Mammalogy Available online at: http://www.italian-journal-of-mammalogy.it doi:10.4404/hystrix–28.2-12362 Commentary The (sleeping) Beauty in the Beast – a review on the water deer, Hydropotes inermis Ann-Marie Schilling1,2,∗, Gertrud E. Rössner1,2,3 1SNSB Bayerische Staatssammlung für Paläontologie und Geologie, Richard-Wagner-Str. 10, 80333 Munich, Germany 2Department of Earth and Environmental Sciences, Ludwig-Maximilians-Universität München, Richard-Wagner-Str. 10, 80333 Munich, Germany 3GeoBio-Center LMU, Ludwig-Maximilians-Universität München, Richard-Wagner-Str. 10, 80333 Munich, Germany Keywords: Abstract biogeography morphology The water deer, Hydropotes inermis (Cervidae, Mammalia), is a small, solitary cervid. It is native to phenotype China and Korea, but some feral populations also live in Europe. In contrast to other deer species, ecology where males are characterized by antlers and small/no upper canines, H. inermis lacks antlers, behaviour genetics but grows long upper canines. For this phenotype and particularities of its biology, the species phylogeny holds considerable potential not only for our understanding of cervid biology, but also for important Cervidae H. inermis fossil record questions about basic developmental and regenerative biology. However, populations conservation are decreasing, and many of the pressing scientific questions motivated by this peculiar species are still open. Here, we review the most different aspects of the species’ biology and discuss scientific publications ranging from the year of the species’ first description in 1870 until 2015. We briefly Article history: sketch its state of conservation, and we discuss the current understanding of its phylogeny. -

Module 4: Negative Strand RNA Viruses Lecture 23: Negative Strand RNA Viruses
NPTEL – Biotechnology – General Virology Module 4: Negative strand RNA viruses Lecture 23: Negative strand RNA viruses Negative strand RNA viruses belong to order Mononegavirales. The viruses in this group have similar genome organization and replication strategies and are probably diverged from a common ancestor (Filoviridae, Paramyxoviridae, Bornaviridae and Rhabdoviridae). They are often associated with emerging infection and havoc to human population (Ebola, Marburg, Nipah and Hendra). Virus contains a negative sense RNA genome which means the polarity of the genome is opposite to that of an mRNA. The negative sense RNA cannot use its genome to synthesize proteins and hence its RNA is not infectious (absence of protein synthesis). Because of the above stated property viruses in this group encode their own polymerase (RNA dependent RNA polymerase [RDRP]). Another unique property about these viruses is about its transcription, first a leader RNA is synthesized, which is followed by sequential transcription of the genes in the 3’ to 5’ order to yield individual mRNAs by a stop-start mechanism guided by the conserved gene-start and gene-end signals. 23.1 Genome features I. Linear non-segmented negative sense RNA genome II. Organization of genome- 3'-Leader-Virion core- Surface proteins-Polymerase- Trailer 5'. III. Helical nucleocapsid contains the RNA dependent RNA polymerase. IV. The leader RNA is neither capped nor polyadenylated and is not functional as mRNA. V. Replication occurs when the polymerase complex ignores the transcription stop signals at the 3’ end of each gene and a full-length positive-sense antigenome is synthesized. VI. Transcription at the gene-start site is not perfect, which leads to a gradient of mRNA abundance that decreases according to the distance from the 3’ end of the genome. -

Evidence to Support Safe Return to Clinical Practice by Oral Health Professionals in Canada During the COVID-19 Pandemic: a Repo
Evidence to support safe return to clinical practice by oral health professionals in Canada during the COVID-19 pandemic: A report prepared for the Office of the Chief Dental Officer of Canada. November 2020 update This evidence synthesis was prepared for the Office of the Chief Dental Officer, based on a comprehensive review under contract by the following: Paul Allison, Faculty of Dentistry, McGill University Raphael Freitas de Souza, Faculty of Dentistry, McGill University Lilian Aboud, Faculty of Dentistry, McGill University Martin Morris, Library, McGill University November 30th, 2020 1 Contents Page Introduction 3 Project goal and specific objectives 3 Methods used to identify and include relevant literature 4 Report structure 5 Summary of update report 5 Report results a) Which patients are at greater risk of the consequences of COVID-19 and so 7 consideration should be given to delaying elective in-person oral health care? b) What are the signs and symptoms of COVID-19 that oral health professionals 9 should screen for prior to providing in-person health care? c) What evidence exists to support patient scheduling, waiting and other non- treatment management measures for in-person oral health care? 10 d) What evidence exists to support the use of various forms of personal protective equipment (PPE) while providing in-person oral health care? 13 e) What evidence exists to support the decontamination and re-use of PPE? 15 f) What evidence exists concerning the provision of aerosol-generating 16 procedures (AGP) as part of in-person -
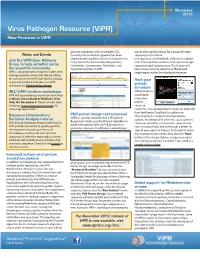
Virus Pathogen Resource (Vipr) New Features in Vipr
November 2012 Virus Pathogen Resource (ViPR) New Features in ViPR genome sequences with incomplete CDS. tool to allow primer design for a group of target News and Events Currently, the annotation pipeline has been sequences in the future . implemented to predict mature viral proteins for In response to user feedback, we have also added Join the ViPR User Advisory many taxa in the Arenaviridae , Bunyaviridae , end-of-line position numbers in the primer design Group to help us better serve Caliciviridae , Coronaviridae , Flaviviridae , and sequence input sequence box. This feature will the scientific community Togaviridae families in ViPR . help ensure accurate selection of the desired ViPR is a bioinformatics resources built for the target region within the displayed sequence. virology research community. We are calling for users to join the ViPR User Advisory Group to provide feedback and advise on ViPR Rock your development. Click here for details . protein structure IRD/ViPR hands-on workshops ViPR provides a ViPR will be providing a hands-on workshop customized at Mount Sinai School of Medicine, New interactive York, NY, December 5 . Please contact Ryan protein Camping ( [email protected] ) for structure workshop registration. viewer for virus-related protein structures obtained from the Protein Data Bank . In addition to PCR primer design tool enhanced choosing from a variety of structure display Sequence Conservation/ ViPR has recently implemented a PCR primer options , including ball & stick, line, space, primary Variation Analysis tutorial design tool , which uses the Primer3 algorithm to The Analyze Sequence Variation (SNP) tool in structure, secondary structure, etc., you can now predict the optimal set(s) of PCR primers for a ViPR provides the ability to quickly quantify rock a structure back and forth to get a better 3D particular sequence . -

The Jungle Times
The Jungle Times Independent newsletter of: Est. 2008 Issue: 113 Inside this issue: Page 7: 10 years of DGFC Page 2: Arrivals Page 3: Visitors Page 5: Departures Page 6: Aberystwyth Field Course Page 7: 10 years of DGFC Page 8: State Action Plans Page 8: State Action Plans Page 9: Science Corner Page 10: Conservation Corner Page 11: Game September 2018 Page 2 Arrivals Jessica Shuttleworth Our final PTY of the year joins us from Oxfordshire. Jess studies Biological Sciences at Cardiff University and is particularly interested in Ecology and Conservation. She has always loved animals since growing up on a pig farm and her love of the outdoors was solidified when trekking through Norway for 3 weeks. She is looking forward to seeing all of Borneo’s amazing wildlife before carrying out her own project here at DG. September 2018 Page 3 Visitors Sabrina Herold Sabrina came from Switzerland to visit for 10 days to experience life at DGFC. She took part in many activities including night walks, primate boats, bird boats and tracking our collared tarsiers. Sabrina also helped to create a welcome pack for future PTYs and volunteers at DG to help future students get more of an idea of the centre before they arrive. Thank you Sabrina! September 2018 Page 4 Visitors – Cardiff University Professor Andrew Weightman, Dr Alison Weightman, Sarah Evans and Professor Susan Baker This month the centre had some visitors from Cardiff University! Professor Andy Weightman from Cardiff School of Biosciences, Sarah Evans, Manager of Cardiff Sustainable Places Research Institute, Professor Susan Baker from Cardiff School of Social Sciences and Dr Alison Weightman visited Borneo this month to attend the ten year anniversary celebration of DGFC.