1 the Distribution of Pond Snail Communities Across A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beiträge Zur Kenntnis Der Paläarktischen Planorbidae. Wilhelm A. Lindholm
Heft 6 Jahrgang LVIII. 1926 Archiv ihr Molluskenkonde Beiträge zur Kenntnis der paläarktischen Planorbidae. Von W. A. Lindholm. I. Zur Synonymie des Planorbis crista (L.). Es ist eine bekannte Tatsache, daß unser kleinster Planorbis hinsichtlich der Skulptur seines Gehäuses verschiedene Varietäten darstellt, welche Eigenschaft ihm im Laufe der Zeiten eine stattliche Anzahl von Artnamen eingebracht hat. Es hatte lange gedauert, bis sich die Forscher überzeugt hatten, daß es sich nicht um mehrere Arten, sondern nur um Formen und z. T. sogar um Wachstumserscheinungen einer einzigen Art handelt. Es kommen eigentlich' nur zwei Formen in Betracht: eine glatte d. h. nur feingestreifte, nicht quer- gerippte, welche meist von den Autoren bis auf den heutigen Tag als PL nautileus (L.) und eine mit ian der Peripherie höckerartig vortretenden Querrippen versehene, welche als PL crista (L.) oder PL cristatus D rap , aufgeführt wird, und deren Jugendtracht an der Peripherie des letzten Umgangs mehr weniger lange Stacheln auf weist. Diese Jugendtracht bewahren ein zelne Individuen auch während ihrer weiteren Lebens dauer; solche Stücke sind von S. CI es sin als PL crista var. spinulosus in die Wissenschaft eingeführt worden. 242 Nun beruht diese landläufige Nomenklatur auf einen Irrtum, da Linné beide erwähnte Artnamen1) auf dasselbe Tier oder richtiger auf dieselbe Abbildung bei Rösel* 2) begründet hatte. Diese Figur stellt nun diejenige Form dar, welche dessin als var. spinulosus bezeichnet hatte, was schon aus RöseFs Worten her vorgeht: ,,Dieses Ammonshorn ist nicht nur gleichsam mit Reifen umleget, sondern es hat auch an seinem Rucken auf jedem Reif eines Stachelspitze.“ Die durch Linné vorgenommene doppelte Benennung dieser Art war seinen Zeitgenossen nicht entgangen, ist aber in unseren Tagen anscheinend vergessen worden. -

Gyraulus Laevis in Nederland 87
Kuijper: Gyraulus laevis in Nederland 87 Gyraulus laevis (Mollusca: Planorbidae) in Nederland door W.J. Kuijper Enkele recente waarnemingen van een van onze zeldzaamste planorbiden waren de aanleiding tot het samenstellen van een over- laevis zicht van alle van Nederland bekende vondsten van Gyraulus (Alder, 1838). Tot voor kort waren slechts enkele vindplaatsen van dit dier bekend (Janssen & De Vogel, 1965: 75). Hierbij komt het betrof feit dat het in een aantal gevallen slechts een enkel exemplaar en de soort niet meer teruggevonden kon worden. Het volgende geeft een chronologisch overzicht van de waarnemingen van Gyraulus laevis in Nederland. Voor zover beschikbaar zijn diverse gegevens van de vindplaatsen vermeld. WAARNEMINGEN 1. Koudekerke (bij Middelburg), buitenplaats Vijvervreugd, ± 1890. Fraai vers materiaal: 69 exemplaren in de collectie Schep- de man (Zoölogisch Museum, Amsterdam). Later niet meer vermeld, buitenplaats bestaat niet meer (Kuiper, 1944: 6). Dit materiaalwerd verzameld door Dr. IJ. Keijzer en is vermeld in: Verslag over 1885-1893 van het Zeeuwsch Genootschap der Wetenschappen, blz. 88 (volgens Mevr. Dr. W.S.S. van der Feen - van Benthem Jut- ting). Verder is materiaal van deze vindplaats in de collecties van het Zeeuwsch Museum te Middelburg (2 ex.) en het Natuurhistorisch Museum te Enschede (6 ex.) aanwezig. 2. Warmond (bij Leiden), in de Leede, januari 1916 (Van Ben- them Jutting, 1933: 179). Dit materiaal werd verzameld door P.P. de Koning; in het Rijksmuseum van Natuurlijke Historie te Leiden bevinden zich vier schelpen van deze vindplaats, waarvan twee een doorsnede van ca. 4 mm bereiken. 3. 't Zand (bij Roodeschool), Oostpolder, 1927 (Van Benthem Jutting, 1947: 66). -

WMSDB - Worldwide Mollusc Species Data Base
WMSDB - Worldwide Mollusc Species Data Base Family: TURBINIDAE Author: Claudio Galli - [email protected] (updated 07/set/2015) Class: GASTROPODA --- Clade: VETIGASTROPODA-TROCHOIDEA ------ Family: TURBINIDAE Rafinesque, 1815 (Sea) - Alphabetic order - when first name is in bold the species has images Taxa=681, Genus=26, Subgenus=17, Species=203, Subspecies=23, Synonyms=411, Images=168 abyssorum , Bolma henica abyssorum M.M. Schepman, 1908 aculeata , Guildfordia aculeata S. Kosuge, 1979 aculeatus , Turbo aculeatus T. Allan, 1818 - syn of: Epitonium muricatum (A. Risso, 1826) acutangulus, Turbo acutangulus C. Linnaeus, 1758 acutus , Turbo acutus E. Donovan, 1804 - syn of: Turbonilla acuta (E. Donovan, 1804) aegyptius , Turbo aegyptius J.F. Gmelin, 1791 - syn of: Rubritrochus declivis (P. Forsskål in C. Niebuhr, 1775) aereus , Turbo aereus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) aethiops , Turbo aethiops J.F. Gmelin, 1791 - syn of: Diloma aethiops (J.F. Gmelin, 1791) agonistes , Turbo agonistes W.H. Dall & W.H. Ochsner, 1928 - syn of: Turbo scitulus (W.H. Dall, 1919) albidus , Turbo albidus F. Kanmacher, 1798 - syn of: Graphis albida (F. Kanmacher, 1798) albocinctus , Turbo albocinctus J.H.F. Link, 1807 - syn of: Littorina saxatilis (A.G. Olivi, 1792) albofasciatus , Turbo albofasciatus L. Bozzetti, 1994 albofasciatus , Marmarostoma albofasciatus L. Bozzetti, 1994 - syn of: Turbo albofasciatus L. Bozzetti, 1994 albulus , Turbo albulus O. Fabricius, 1780 - syn of: Menestho albula (O. Fabricius, 1780) albus , Turbo albus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) albus, Turbo albus T. Pennant, 1777 amabilis , Turbo amabilis H. Ozaki, 1954 - syn of: Bolma guttata (A. Adams, 1863) americanum , Lithopoma americanum (J.F. -
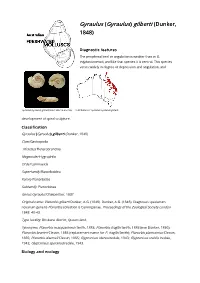
Gyraulus) Gilberti (Dunker, 1848
Gyraulus (Gyraulus) gilberti (Dunker, 1848) Diagnostic features The peripheral keel or angulation is weaker than in G. edgbastonensis, and like that species it is central. This species varies widely in degree of depression and angulation, and Gyraulus (Gyraulus) gilberti (adult size 4.8-5.5 mm) Distribution of Gyraulus (Gyraulus) gilberti. development of spiral sculpture. Classification Gyraulus (Gyraulus) gilberti (Dunker, 1848) Class Gastropoda I nfraclass Heterobranchia Megaorder Hygrophila Order Lymnaeida Superfamily Planorboidea Family Planorbidae Subfamily: Planorbinae Genus Gyraulus Charpentier, 1837 Original name: Planorbis gilberti Dunker, A.G. (1848). Dunker, A.G. (1848). Diagnoses specierum novarum generis Planorbis collection is Cumingianae. Proceedings of the Zoological Society London 1848: 40-43. Type locality: Brisbane district, Queensland. Synonyms: Planorbis macquariensis Smith, 1883; Planorbis fragilis Smith, 1883 (non Dunker, 1850); Planorbis brazieri Clessin, 1885 (replacement name for P. fragilis Smith); Planorbis planissimus Clessin, 1885; Planorbis daemeli Clessin, 1885; Glyptanisus idenusredale, 1943; Glyptanisus stabilis redale, 1943; Glyptanisus speranusredale, 1943. Biology and ecology This species lives in water weeds and other vegetation in ponds, billabongs, swamps and sluggish streams and rivers in tropical and subtropical eastern Australia. Feeds on detritus. Egg mass presumably a jelly strip containing small eggs. Development direct. Brown (2001) described the anatomy of this species. This species is an intermediate host for the stomach fluke Orthocoelium streptocoelium (Boray, 1982; Beesley et al., 1998). Distribution This species occurs throughout eastern Australia, from Cape York to northern New South Wales. Notes G. isingi and/or G.waterhousei may possibly be conspecific with this species (Brown, 2001). Further reading Beesley, P. L., Ross, G. J. -

Anisus Vorticulus (Troschel 1834) (Gastropoda: Planorbidae) in Northeast Germany
JOURNAL OF CONCHOLOGY (2013), VOL.41, NO.3 389 SOME ECOLOGICAL PECULIARITIES OF ANISUS VORTICULUS (TROSCHEL 1834) (GASTROPODA: PLANORBIDAE) IN NORTHEAST GERMANY MICHAEL L. ZETTLER Leibniz Institute for Baltic Sea Research Warnemünde, Seestr. 15, D-18119 Rostock, Germany Abstract During the EU Habitats Directive monitoring between 2008 and 2010 the ecological requirements of the gastropod species Anisus vorticulus (Troschel 1834) were investigated in 24 different waterbodies of northeast Germany. 117 sampling units were analyzed quantitatively. 45 of these units contained living individuals of the target species in abundances between 4 and 616 individuals m-2. More than 25.300 living individuals of accompanying freshwater mollusc species and about 9.400 empty shells were counted and determined to the species level. Altogether 47 species were identified. The benefit of enhanced knowledge on the ecological requirements was gained due to the wide range and high number of sampled habitats with both obviously convenient and inconvenient living conditions for A. vorticulus. In northeast Germany the amphibian zones of sheltered mesotrophic lake shores, swampy (lime) fens and peat holes which are sun exposed and have populations of any Chara species belong to the optimal, continuously and densely colonized biotopes. The cluster analysis emphasized that A. vorticulus was associated with a typical species composition, which can be named as “Anisus-vorticulus-community”. In compliance with that both the frequency of combined occurrence of species and their similarity in relative abundance are important. The following species belong to the “Anisus-vorticulus-community” in northeast Germany: Pisidium obtusale, Pisidium milium, Pisidium pseudosphaerium, Bithynia leachii, Stagnicola palustris, Valvata cristata, Bathyomphalus contortus, Bithynia tentaculata, Anisus vortex, Hippeutis complanatus, Gyraulus crista, Physa fontinalis, Segmentina nitida and Anisus vorticulus. -

Invertebrate Animals (Metazoa: Invertebrata) of the Atanasovsko Lake, Bulgaria
Historia naturalis bulgarica, 22: 45-71, 2015 Invertebrate Animals (Metazoa: Invertebrata) of the Atanasovsko Lake, Bulgaria Zdravko Hubenov, Lyubomir Kenderov, Ivan Pandourski Abstract: The role of the Atanasovsko Lake for storage and protection of the specific faunistic diversity, characteristic of the hyper-saline lakes of the Bulgarian seaside is presented. The fauna of the lake and surrounding waters is reviewed, the taxonomic diversity and some zoogeographical and ecological features of the invertebrates are analyzed. The lake system includes from freshwater to hyper-saline basins with fast changing environment. A total of 6 types, 10 classes, 35 orders, 82 families and 157 species are known from the Atanasovsko Lake and the surrounding basins. They include 56 species (35.7%) marine and marine-brackish forms and 101 species (64.3%) brackish-freshwater, freshwater and terrestrial forms, connected with water. For the first time, 23 species in this study are established (12 marine, 1 brackish and 10 freshwater). The marine and marine- brackish species have 4 types of ranges – Cosmopolitan, Atlantic-Indian, Atlantic-Pacific and Atlantic. The Atlantic (66.1%) and Cosmopolitan (23.2%) ranges that include 80% of the species, predominate. Most of the fauna (over 60%) has an Atlantic-Mediterranean origin and represents an impoverished Atlantic-Mediterranean fauna. The freshwater-brackish, freshwater and terrestrial forms, connected with water, that have been established from the Atanasovsko Lake, have 2 main types of ranges – species, distributed in the Palaearctic and beyond it and species, distributed only in the Palaearctic. The representatives of the first type (52.4%) predomi- nate. They are related to the typical marine coastal habitats, optimal for the development of certain species. -

Freshwater Snail Guide
A Beginner’s Guide to Freshwater Snails of Central Scotland Freshwater snails are a diverse and fascinating group of molluscs found in ponds, lochs, canals, rivers and watery ditches all across the globe. This guide is a simple introduction to some of the most commonly encountered and easily identifiable freshwater snail species in Central Scotland. How do I look for freshwater snails? 1) Choose your spot: anywhere with water – your garden pond, a nearby river, canal or loch – is likely to have snails. 2) Grab a pond net (buy online or make your own by attaching a sieve to the end of an old broom or mop), a white tray or tub and a hand-lens or magnifier. 3) Fill the tray with water from your chosen habitat. Sweep the net around in the water, making sure to get among any vegetation (don’t go too near the bottom or you’ll get a net full of mud), empty your net into the water-filled tray and identify what you have found! Snail shell features Spire Whorls Some snail species have a hard plate called an ‘operculum’ Height which they Mouth (Aperture) use to seal the mouth of the shell when they are inside Height Pointed shell Flat shell Width Width Pond Snails (Lymnaeidae) Variable in size. Mouth always on right-hand side, shells usually long and pointed. Great Pond Snail Common Pond Snail Lymnaea stagnalis Radix balthica Largest pond snail. Common in ponds Fairly rounded and ’fat’. Common in weedy lakes, canals and sometimes slow river still waters. pools. -

Folk Taxonomy, Nomenclature, Medicinal and Other Uses, Folklore, and Nature Conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3
Ulicsni et al. Journal of Ethnobiology and Ethnomedicine (2016) 12:47 DOI 10.1186/s13002-016-0118-7 RESEARCH Open Access Folk knowledge of invertebrates in Central Europe - folk taxonomy, nomenclature, medicinal and other uses, folklore, and nature conservation Viktor Ulicsni1* , Ingvar Svanberg2 and Zsolt Molnár3 Abstract Background: There is scarce information about European folk knowledge of wild invertebrate fauna. We have documented such folk knowledge in three regions, in Romania, Slovakia and Croatia. We provide a list of folk taxa, and discuss folk biological classification and nomenclature, salient features, uses, related proverbs and sayings, and conservation. Methods: We collected data among Hungarian-speaking people practising small-scale, traditional agriculture. We studied “all” invertebrate species (species groups) potentially occurring in the vicinity of the settlements. We used photos, held semi-structured interviews, and conducted picture sorting. Results: We documented 208 invertebrate folk taxa. Many species were known which have, to our knowledge, no economic significance. 36 % of the species were known to at least half of the informants. Knowledge reliability was high, although informants were sometimes prone to exaggeration. 93 % of folk taxa had their own individual names, and 90 % of the taxa were embedded in the folk taxonomy. Twenty four species were of direct use to humans (4 medicinal, 5 consumed, 11 as bait, 2 as playthings). Completely new was the discovery that the honey stomachs of black-coloured carpenter bees (Xylocopa violacea, X. valga)were consumed. 30 taxa were associated with a proverb or used for weather forecasting, or predicting harvests. Conscious ideas about conserving invertebrates only occurred with a few taxa, but informants would generally refrain from harming firebugs (Pyrrhocoris apterus), field crickets (Gryllus campestris) and most butterflies. -

A Ma Aeolake in Alacologi N the Moe Cal Analy
Faculty of Sciences Department of Geology and Soil Science Research Unit Palaeontology Academic year 2009‐2010 Changes in surface waters: a malacological analysis of a Late Glacial and early Holocene palaeolake in the Moervaartdepression (Belgium). by Lynn Serbruyns Thesis submitted to obtain the degree of Master in Biology. Promotor: Prof. Dr. Jacques Verniers Co‐promotor: Prof. Dr. Dirk Van Damme Faculty of Sciences Department of Geology and Soil Science Research Unit Palaeontology Academic year 2009‐2010 Changes in surface waters: a malacological analysis of a Late Glacial and early Holocene palaeolake in the Moervaartdepression (Belgium). by Lynn Serbruyns Thesis submitted to obtain the degree of Master in Biology. Promotor: Prof. Dr. Jacques Verniers Co‐promotor: Prof. Dr. Dirk Van Damme Acknowledgements0 First of all, I would like to thank my promoter Prof. Jacques Verniers and Prof. Philippe Crombé for providing me with this interesting subject and for giving me the freedom to further extend the analysis beyond the original boundaries. Thanks to my co-promoter Prof. Dirk Van Damme who I could always contact with questions and who provided me with many articles on the subject. I also want to thank Prof. Keppens for giving me the opportunity to perform the isotope analysis at the VUB, even though technology let us down in the end. I would like to thank Koen Verhoeven for sacrificing part of his office and for aiding me with the sampling from the trench. Thanks to Mona Court-Picon for the numerous ways in which she helped me during the making of this thesis and for the nice talks. -

Aquatic Snails of the Snake and Green River Basins of Wyoming
Aquatic snails of the Snake and Green River Basins of Wyoming Lusha Tronstad Invertebrate Zoologist Wyoming Natural Diversity Database University of Wyoming 307-766-3115 [email protected] Mark Andersen Information Systems and Services Coordinator Wyoming Natural Diversity Database University of Wyoming 307-766-3036 [email protected] Suggested citation: Tronstad, L.M. and M. D. Andersen. 2018. Aquatic snails of the Snake and Green River Basins of Wyoming. Report prepared by the Wyoming Natural Diversity Database for the Wyoming Fish and Wildlife Department. 1 Abstract Freshwater snails are a diverse group of mollusks that live in a variety of aquatic ecosystems. Many snail species are of conservation concern around the globe. About 37-39 species of aquatic snails likely live in Wyoming. The current study surveyed the Snake and Green River basins in Wyoming and identified 22 species and possibly discovered a new operculate snail. We surveyed streams, wetlands, lakes and springs throughout the basins at randomly selected locations. We measured habitat characteristics and basic water quality at each site. Snails were usually most abundant in ecosystems with higher standing stocks of algae, on solid substrate (e.g., wood or aquatic vegetation) and in habitats with slower water velocity (e.g., backwater and margins of streams). We created an aquatic snail key for identifying species in Wyoming. The key is a work in progress that will be continually updated to reflect changes in taxonomy and new knowledge. We hope the snail key will be used throughout the state to unify snail identification and create better data on Wyoming snails. -

Final Reports of the Tibor T. Polgar Fellowship Program, 2018
REPORTS OF THE TIBOR T. POLGAR FELLOWSHIP PROGRAM, 2018 Sarah H. Fernald, David J. Yozzo, and Helena Andreyko Editors A Joint Program of The Hudson River Foundation and The New York State Department of Environmental Conservation December 2020 i ii ABSTRACT Eight studies completed within the Hudson River Estuary under the auspices of the Tibor T. Polgar Fellowship Program during 2018 have been included in the current volume. Major objectives of these studies included: (1) determining the effects of light, nutrients, and temperature on cyanobacterial blooms, (2) quantifying the differences in microplastic concentrations among marsh, tributary, and open water locations in the Hudson River watershed, (3) determining the effect of microplastic size and shape on the uptake ability of the Eastern Oyster (Crassostrea virginia), (4) evaluating the effect of salinity on gametogenesis in Eastern Oysters in the Hudson River, (5) determining the effect of the redox environment on anaerobic biodegradability of personal care products by native microorganisms in anoxic estuarine sediments, (6) comparing Vallisneria americana reproduction modes between sites in the Hudson River and Chesapeake Bay, (7) characterizing habitat use of tidal wetlands by the painted turtle (Chrysemys picta), and (8) using core sample analysis to determine the environmental history of Ramshorn- Livingston Marsh. iii iv TABLE OF CONTENTS Abstract ............................................................................................................... iii Preface ................................................................................................................ -

First Record of the Acute Bladder Snail Physella Acuta (Draparnaud, 1805) in the Wild Waters of Lithuania
BioInvasions Records (2019) Volume 8, Issue 2: 281–286 CORRECTED PROOF Rapid Communication First record of the acute bladder snail Physella acuta (Draparnaud, 1805) in the wild waters of Lithuania Rokas Butkus1,*, Giedrė Višinskienė2 and Kęstutis Arbačiauskas2,3 1Marine Research Institute, Klaipėda University, Herkaus Manto Str. 84, LT-92294 Klaipėda, Lithuania 2Nature Research Centre, Akademijos str. 2, LT-08412 Vilnius, Lithuania 3Department of Zoology, Institute of Biosciences, Vilnius University, Saulėtekio al. 7, LT-10257 Vilnius, Lithuania *Corresponding author E-mail: [email protected] Citation: Butkus R, Višinskienė G, Arbačiauskas K (2019) First record of the Abstract acute bladder snail Physella acuta (Draparnaud, 1805) in the wild waters of The acute bladder snail Physella acuta (Draparnaud, 1805) was observed for the Lithuania. BioInvasions Records 8(2): first time in the wild waters of Lithuania at one site in the lower reaches of the 281–286, https://doi.org/10.3391/bir.2019.8.2.10 Nevėžis River in 2015. The restricted distribution and low density suggest recent th Received: 11 October 2018 introduction. Although P. acuta in the first half of the 20 century was reported in Accepted: 25 February 2019 ponds of the Kaunas Botanical Garden, they appear to have vanished as of 2012. Thus we conclude that recent invasion into the wild most probably has resulted Published: 29 April 2019 from disposal of aquarium organisms. Thematic editor: David Wong Copyright: © Butkus et al. Key words: aquarium trade, local distribution, recent introduction, river This is an open access article distributed under terms of the Creative Commons Attribution License (Attribution 4.0 International - CC BY 4.0).