(Gastropoda) with a Rhipidoglossate Radula
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Naturkunde-Museum Bamberg (NKMB)
Jahresbericht 2008 der Generaldirektion der Staatlichen Naturwissenschaftlichen Sammlungen Bayerns Herausgegeben von: Prof. Dr. Gerhard Haszprunar Generaldirektor Der Staatlichen Naturwissenschaftlichen Sammlungen Bayerns Menzinger Straße 71, 80638 München München November 2009 Zusammenstellung und Endredaktion: Dr. Eva Maria Natzer (Generaldirektion) Unterstützung durch: Maria-Luise Kaim (Generaldirektion) Susanne Legat (Generaldirektion) Dr. Jörg Spelda (Generaldirektion) Druck: Digitaldruckzentrum, Amalienstrasse, München Inhaltsverzeichnis Bericht des Generaldirektors ...................................................................................................5 Wissenschaftliche Publikationen ................................................................................................7 Drittmittelübersicht ...................................................................................................................36 Organigramm ............................................................................................................................51 Generaldirektion .....................................................................................................................52 Personalvertretung ....................................................................................................................54 Museen Museum Mensch und Natur (MMN) ........................................................................................55 Museum Reich der Kristalle (MRK) ........................................................................................61 -

Gyraulus Laevis in Nederland 87
Kuijper: Gyraulus laevis in Nederland 87 Gyraulus laevis (Mollusca: Planorbidae) in Nederland door W.J. Kuijper Enkele recente waarnemingen van een van onze zeldzaamste planorbiden waren de aanleiding tot het samenstellen van een over- laevis zicht van alle van Nederland bekende vondsten van Gyraulus (Alder, 1838). Tot voor kort waren slechts enkele vindplaatsen van dit dier bekend (Janssen & De Vogel, 1965: 75). Hierbij komt het betrof feit dat het in een aantal gevallen slechts een enkel exemplaar en de soort niet meer teruggevonden kon worden. Het volgende geeft een chronologisch overzicht van de waarnemingen van Gyraulus laevis in Nederland. Voor zover beschikbaar zijn diverse gegevens van de vindplaatsen vermeld. WAARNEMINGEN 1. Koudekerke (bij Middelburg), buitenplaats Vijvervreugd, ± 1890. Fraai vers materiaal: 69 exemplaren in de collectie Schep- de man (Zoölogisch Museum, Amsterdam). Later niet meer vermeld, buitenplaats bestaat niet meer (Kuiper, 1944: 6). Dit materiaalwerd verzameld door Dr. IJ. Keijzer en is vermeld in: Verslag over 1885-1893 van het Zeeuwsch Genootschap der Wetenschappen, blz. 88 (volgens Mevr. Dr. W.S.S. van der Feen - van Benthem Jut- ting). Verder is materiaal van deze vindplaats in de collecties van het Zeeuwsch Museum te Middelburg (2 ex.) en het Natuurhistorisch Museum te Enschede (6 ex.) aanwezig. 2. Warmond (bij Leiden), in de Leede, januari 1916 (Van Ben- them Jutting, 1933: 179). Dit materiaal werd verzameld door P.P. de Koning; in het Rijksmuseum van Natuurlijke Historie te Leiden bevinden zich vier schelpen van deze vindplaats, waarvan twee een doorsnede van ca. 4 mm bereiken. 3. 't Zand (bij Roodeschool), Oostpolder, 1927 (Van Benthem Jutting, 1947: 66). -
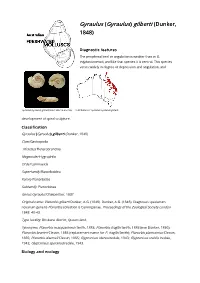
Gyraulus) Gilberti (Dunker, 1848
Gyraulus (Gyraulus) gilberti (Dunker, 1848) Diagnostic features The peripheral keel or angulation is weaker than in G. edgbastonensis, and like that species it is central. This species varies widely in degree of depression and angulation, and Gyraulus (Gyraulus) gilberti (adult size 4.8-5.5 mm) Distribution of Gyraulus (Gyraulus) gilberti. development of spiral sculpture. Classification Gyraulus (Gyraulus) gilberti (Dunker, 1848) Class Gastropoda I nfraclass Heterobranchia Megaorder Hygrophila Order Lymnaeida Superfamily Planorboidea Family Planorbidae Subfamily: Planorbinae Genus Gyraulus Charpentier, 1837 Original name: Planorbis gilberti Dunker, A.G. (1848). Dunker, A.G. (1848). Diagnoses specierum novarum generis Planorbis collection is Cumingianae. Proceedings of the Zoological Society London 1848: 40-43. Type locality: Brisbane district, Queensland. Synonyms: Planorbis macquariensis Smith, 1883; Planorbis fragilis Smith, 1883 (non Dunker, 1850); Planorbis brazieri Clessin, 1885 (replacement name for P. fragilis Smith); Planorbis planissimus Clessin, 1885; Planorbis daemeli Clessin, 1885; Glyptanisus idenusredale, 1943; Glyptanisus stabilis redale, 1943; Glyptanisus speranusredale, 1943. Biology and ecology This species lives in water weeds and other vegetation in ponds, billabongs, swamps and sluggish streams and rivers in tropical and subtropical eastern Australia. Feeds on detritus. Egg mass presumably a jelly strip containing small eggs. Development direct. Brown (2001) described the anatomy of this species. This species is an intermediate host for the stomach fluke Orthocoelium streptocoelium (Boray, 1982; Beesley et al., 1998). Distribution This species occurs throughout eastern Australia, from Cape York to northern New South Wales. Notes G. isingi and/or G.waterhousei may possibly be conspecific with this species (Brown, 2001). Further reading Beesley, P. L., Ross, G. J. -

Ringiculid Bubble Snails Recovered As the Sister Group to Sea Slugs
www.nature.com/scientificreports OPEN Ringiculid bubble snails recovered as the sister group to sea slugs (Nudipleura) Received: 13 May 2016 Yasunori Kano1, Bastian Brenzinger2,3, Alexander Nützel4, Nerida G. Wilson5 & Accepted: 08 July 2016 Michael Schrödl2,3 Published: 08 August 2016 Euthyneuran gastropods represent one of the most diverse lineages in Mollusca (with over 30,000 species), play significant ecological roles in aquatic and terrestrial environments and affect many aspects of human life. However, our understanding of their evolutionary relationships remains incomplete due to missing data for key phylogenetic lineages. The present study integrates such a neglected, ancient snail family Ringiculidae into a molecular systematics of Euthyneura for the first time, and is supplemented by the first microanatomical data. Surprisingly, both molecular and morphological features present compelling evidence for the common ancestry of ringiculid snails with the highly dissimilar Nudipleura—the most species-rich and well-known taxon of sea slugs (nudibranchs and pleurobranchoids). A new taxon name Ringipleura is proposed here for these long-lost sisters, as one of three major euthyneuran clades with late Palaeozoic origins, along with Acteonacea (Acteonoidea + Rissoelloidea) and Tectipleura (Euopisthobranchia + Panpulmonata). The early Euthyneura are suggested to be at least temporary burrowers with a characteristic ‘bubble’ shell, hypertrophied foot and headshield as exemplified by many extant subtaxa with an infaunal mode of life, while the expansion of the mantle might have triggered the explosive Mesozoic radiation of the clade into diverse ecological niches. The traditional gastropod subclass Euthyneura is a highly diverse clade of snails and slugs with at least 30,000 living species1,2. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Mollusca, Archaeogastropoda) from the Northeastern Pacific
Zoologica Scripta, Vol. 25, No. 1, pp. 35-49, 1996 Pergamon Elsevier Science Ltd © 1996 The Norwegian Academy of Science and Letters Printed in Great Britain. All rights reserved 0300-3256(95)00015-1 0300-3256/96 $ 15.00 + 0.00 Anatomy and systematics of bathyphytophilid limpets (Mollusca, Archaeogastropoda) from the northeastern Pacific GERHARD HASZPRUNAR and JAMES H. McLEAN Accepted 28 September 1995 Haszprunar, G. & McLean, J. H. 1995. Anatomy and systematics of bathyphytophilid limpets (Mollusca, Archaeogastropoda) from the northeastern Pacific.—Zool. Scr. 25: 35^9. Bathyphytophilus diegensis sp. n. is described on basis of shell and radula characters. The radula of another species of Bathyphytophilus is illustrated, but the species is not described since the shell is unknown. Both species feed on detached blades of the surfgrass Phyllospadix carried by turbidity currents into continental slope depths in the San Diego Trough. The anatomy of B. diegensis was investigated by means of semithin serial sectioning and graphic reconstruction. The shell is limpet like; the protoconch resembles that of pseudococculinids and other lepetelloids. The radula is a distinctive, highly modified rhipidoglossate type with close similarities to the lepetellid radula. The anatomy falls well into the lepetelloid bauplan and is in general similar to that of Pseudococculini- dae and Pyropeltidae. Apomorphic features are the presence of gill-leaflets at both sides of the pallial roof (shared with certain pseudococculinids), the lack of jaws, and in particular many enigmatic pouches (bacterial chambers?) which open into the posterior oesophagus. Autapomor- phic characters of shell, radula and anatomy confirm the placement of Bathyphytophilus (with Aenigmabonus) in a distinct family, Bathyphytophilidae Moskalev, 1978. -

Malacologia, 1993, 35(2); 261-313
^;^2_ MALACOLOGIA, 1993, 35(2); 261-313 PHYLOGENETIC RELATIONSHIPS AND GENERIC REVIEW OF THE BITTIINAE (PROSOBRANCHIA: GERITHIOIDEA) Richard S. Houbrick Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560, U.S.A. ABSTRACT The anatomy of seven members of the Bittium group is described, clarifying the status of the genus-level taxa comprising it. Bittium reticulatum, the type species of Bittium Gray, is described in depth, thereby establishing criteria for comparisons with other taxa of Bitliinae. The type species of Stylidium Dell and LirobiWum Bartsch, and representatives of Bittiolum Cossmann and Cacozeliana Strand are examined and compared with Bittium, s.s. Results of anatomical studies and a phylogenetic analysis using the Hennig86 and CLADOS programs, with Cehtt)ium as an outgroup, establish monophyly for Bitliinae Cossmann and reveal six different genus-level taxa. A new genus, ittibittium, from the Indo-Pacific, is proposed. Synonymies of each genus- level taxon and representative species examined are presented. Brief accounts of the ecology and zoogeography of each taxon are given. Two taxa formerly attributed to the 6/ff/um-group are herein excluded from it and referred to Cerithium Bruguière. These are Cerithium zebrum Kiener, 1841, and Cerithium boeticum Pease, 1861. The subfamily Bittiinae Cossmann, 1906, is thought to comprise nine genera (four of which were not included in phylogenetic analyses) : Bittium Gray, 1847; Bittiolum Cossmann, 1906; Ittibittium gen. n., Stylidium Dalí, 1907; Lirobit- tium Bartsch, 1911 ; Cacozeliana Strand, 1928; Argyropeza Melvill & Standen, 1901 ; Varicopeza Gründel, 1976; Zebittium Finlay, 1927. The genus Cassiella Gofas, 1987, of uncertain place- ment, is included as a possible member of the group. -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

Assessment of Mitochondrial Genomes for Heterobranch Gastropod Phylogenetics
Assessment of mitochondrial genomes for heterobranch gastropod phylogenetics Rebecca M Varney University of Alabama Bastian Brenzinger Staatliche Naturwissenschaftliche Sammlungen Bayerns Manuel António E. Malaquias Universitetsmuseet i Bergen Christopher P. Meyer Smithsonian Institution Michael Schrödl Staatliche Naturwissenschaftliche Sammlungen Bayerns Kevin Kocot ( [email protected] ) The University of Alabama https://orcid.org/0000-0002-8673-2688 Research article Keywords: Heterobranchia, Gastropoda, mitochondrial genome, mitogenomic Posted Date: December 10th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-30542/v3 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on January 21st, 2021. See the published version at https://doi.org/10.1186/s12862-020-01728-y. Page 1/19 Abstract Background Heterobranchia is a diverse clade of marine, freshwater, and terrestrial gastropod molluscs. It includes such disparate taxa as nudibranchs, sea hares, bubble snails, pulmonate land snails and slugs, and a number of (mostly small-bodied) poorly known snails and slugs collectively referred to as the “lower heterobranchs.” Evolutionary relationships within Heterobranchia have been challenging to resolve and the group has been subject to frequent and signicant taxonomic revision. Mitochondrial (mt) genomes can be a useful molecular marker for phylogenetics but, to date, sequences have been available for only a relatively small subset of Heterobranchia. Results To assess the utility of mitochondrial genomes for resolving evolutionary relationships within this clade, eleven new mt genomes were sequenced including representatives of several groups of “lower heterobranchs.” Maximum likelihood analyses of concatenated matrices of the thirteen protein coding genes found weak support for most higher-level relationships even after several taxa with extremely high rates of evolution were excluded. -

Utility of H3-Genesequences for Phylogenetic Reconstruction – a Case Study of Heterobranch Gastropoda –*
Bonner zoologische Beiträge Band 55 (2006) Heft 3/4 Seiten 191–202 Bonn, November 2007 Utility of H3-Genesequences for phylogenetic reconstruction – a case study of heterobranch Gastropoda –* Angela DINAPOLI1), Ceyhun TAMER1), Susanne FRANSSEN1), Lisha NADUVILEZHATH1) & Annette KLUSSMANN-KOLB1) 1)Department of Ecology, Evolution and Diversity – Phylogeny and Systematics, J. W. Goethe-University, Frankfurt am Main, Germany *Paper presented to the 2nd International Workshop on Opisthobranchia, ZFMK, Bonn, Germany, September 20th to 22nd, 2006 Abstract. In the present study we assessed the utility of H3-Genesequences for phylogenetic reconstruction of the He- terobranchia (Mollusca, Gastropoda). Therefore histone H3 data were collected for 49 species including most of the ma- jor groups. The sequence alignment provided a total of 246 sites of which 105 were variable and 96 parsimony informa- tive. Twenty-four (of 82) first base positions were variable as were 78 of the third base positions but only 3 of the se- cond base positions. H3 analyses showed a high codon usage bias. The consistency index was low (0,210) and a substitution saturation was observed in the 3r d codon position. The alignment with the translation of the H3 DNA sequences to amino-acid sequences had no sites that were parsimony-informative within the Heterobranchia. Phylogenetic trees were reconstructed using maximum parsimony, maximum likelihood and Bayesian methodologies. Nodilittorina unifasciata was used as outgroup. The resolution of the deeper nodes was limited in this molecular study. The data themselves were not sufficient to clar- ify phylogenetic relationships within Heterobranchia. Neither the monophyly of the Euthyneura nor a step-by-step evo- lution by the “basal” groups was supported. -

Caenogastropoda
13 Caenogastropoda Winston F. Ponder, Donald J. Colgan, John M. Healy, Alexander Nützel, Luiz R. L. Simone, and Ellen E. Strong Caenogastropods comprise about 60% of living Many caenogastropods are well-known gastropod species and include a large number marine snails and include the Littorinidae (peri- of ecologically and commercially important winkles), Cypraeidae (cowries), Cerithiidae (creep- marine families. They have undergone an ers), Calyptraeidae (slipper limpets), Tonnidae extraordinary adaptive radiation, resulting in (tuns), Cassidae (helmet shells), Ranellidae (tri- considerable morphological, ecological, physi- tons), Strombidae (strombs), Naticidae (moon ological, and behavioral diversity. There is a snails), Muricidae (rock shells, oyster drills, etc.), wide array of often convergent shell morpholo- Volutidae (balers, etc.), Mitridae (miters), Buccin- gies (Figure 13.1), with the typically coiled shell idae (whelks), Terebridae (augers), and Conidae being tall-spired to globose or fl attened, with (cones). There are also well-known freshwater some uncoiled or limpet-like and others with families such as the Viviparidae, Thiaridae, and the shells reduced or, rarely, lost. There are Hydrobiidae and a few terrestrial groups, nota- also considerable modifi cations to the head- bly the Cyclophoroidea. foot and mantle through the group (Figure 13.2) Although there are no reliable estimates and major dietary specializations. It is our aim of named species, living caenogastropods are in this chapter to review the phylogeny of this one of the most diverse metazoan clades. Most group, with emphasis on the areas of expertise families are marine, and many (e.g., Strombidae, of the authors. Cypraeidae, Ovulidae, Cerithiopsidae, Triphori- The fi rst records of undisputed caenogastro- dae, Olividae, Mitridae, Costellariidae, Tereb- pods are from the middle and upper Paleozoic, ridae, Turridae, Conidae) have large numbers and there were signifi cant radiations during the of tropical taxa. -

From the Late Triassic St Cassian Formation
Mathildoidea (Gastropoda, Heterostropha) from the Late Triassic St Cassian Formation Klaus Bandel Bandel, Κ. Mathildoidea (Gastropoda, Heterostropha) from the Late Triassic St Cassian Formation. — Scripta Geol., Ill: 1-83,19 pls, Leiden, November 1995. Klaus Bandel, Geologisch-Paläontologisches Institut, Universität Hamburg, Bundesstraße 55, D-20146 Hamburg, Germany. Key words: Gastropoda, Heterostropha, Late Triassic, evolution. In the St Cassian fauna of Late Triassic (Early Carnian) age gastropods with protoconch coiled in opposite direction to the teleoconch are common and belong to a number of quite different taxa. Twenty nine of these are here described, 11 of them for the first time: Promathilda misurinensis sp. nov., Turrithilda cassiana sp. nov., T. dockeryi sp. nov., Tirolthilda seelandica gen. et sp. nov., T. nuetzeli sp. nov., Tofanella cancellata sp. nov., Cristalloella cassiana gen. et sp. nov., C. sinuata sp. nov., C. delicata sp. nov., Stuorilda cassiana gen. et sp. nov., and S. tichyi sp. nov. All are newly defined and placed in the Mathildoidea. This connects the Triassic species of that superfamity with the modern Heterostropha (= Heterobranchia). In the family Mathildidae the genera Mathilda and Promathilda are differentiated, two species of Turrithilda described, and Tirolthilda and Schroederilda are included as new genera, with the type species T. seelandica gen. et sp. nov. and Pseudotritonium millierense Zardini, 1978, respectively. The new family Anoptychiidae holds the genera Anoptychia, Turristylus and Camponella gen. nov. (type species Coelostylina pianozensis Zardini, 1985). Here the juvenile ornament resembles that of the Mathildidae but differs from the later smooth teleoconch. Protoconch morphology differentiates the new families Tofanellidae, Trachoecidae and Ampezzanildidae. In contrast to the Mathildidae, Dolomitellidae and Anoptychiidae, the sinistral shell of the protoconch changes its direction of coiling within the larval part of the shell and not at the transition from larval shell to teleoconch.