Prerequisites for Flying Snails: External Transport Potential of Aquatic Snails by Waterbirds Author(S): C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic
water Review Fresh- and Brackish-Water Cold-Tolerant Species of Southern Europe: Migrants from the Paratethys That Colonized the Arctic Valentina S. Artamonova 1, Ivan N. Bolotov 2,3,4, Maxim V. Vinarski 4 and Alexander A. Makhrov 1,4,* 1 A. N. Severtzov Institute of Ecology and Evolution, Russian Academy of Sciences, 119071 Moscow, Russia; [email protected] 2 Laboratory of Molecular Ecology and Phylogenetics, Northern Arctic Federal University, 163002 Arkhangelsk, Russia; [email protected] 3 Federal Center for Integrated Arctic Research, Russian Academy of Sciences, 163000 Arkhangelsk, Russia 4 Laboratory of Macroecology & Biogeography of Invertebrates, Saint Petersburg State University, 199034 Saint Petersburg, Russia; [email protected] * Correspondence: [email protected] Abstract: Analysis of zoogeographic, paleogeographic, and molecular data has shown that the ancestors of many fresh- and brackish-water cold-tolerant hydrobionts of the Mediterranean region and the Danube River basin likely originated in East Asia or Central Asia. The fish genera Gasterosteus, Hucho, Oxynoemacheilus, Salmo, and Schizothorax are examples of these groups among vertebrates, and the genera Magnibursatus (Trematoda), Margaritifera, Potomida, Microcondylaea, Leguminaia, Unio (Mollusca), and Phagocata (Planaria), among invertebrates. There is reason to believe that their ancestors spread to Europe through the Paratethys (or the proto-Paratethys basin that preceded it), where intense speciation took place and new genera of aquatic organisms arose. Some of the forms that originated in the Paratethys colonized the Mediterranean, and overwhelming data indicate that Citation: Artamonova, V.S.; Bolotov, representatives of the genera Salmo, Caspiomyzon, and Ecrobia migrated during the Miocene from I.N.; Vinarski, M.V.; Makhrov, A.A. -

A Note on Oviposition by Lymnaea Stagnalis (Linnaeus, 1758) (Gastropoda: Pulmonata: Lymnaeidae) on Shells of Conspecifics Under Laboratory Conditions
Folia Malacol. 25(2): 101–108 https://doi.org/10.12657/folmal.025.007 A NOTE ON OVIPOSITION BY LYMNAEA STAGNALIS (LINNAEUS, 1758) (GASTROPODA: PULMONATA: LYMNAEIDAE) ON SHELLS OF CONSPECIFICS UNDER LABORATORY CONDITIONS PAOLA LOMBARDO1*, FRANCESCO PAOLO MICCOLI2 1 Limno Consulting, via Bedollo 303, I-00124 Rome, Italy (e-mail: [email protected]) 2 University of L’Aquila, Coppito Science Center, I-67100 L’Aquila, Italy (e-mail: [email protected]) *corresponding author ABSTRACT: Oviposition by Lymnaea stagnalis (L.) on shells of conspecifics has been reported anecdotally from laboratory observations. In order to gain the first quantitative insight into this behaviour, we have quantified the proportion of individuals bearing egg clutches in a long-term monospecific outdoor laboratory culture of L. stagnalis during two consecutive late-summer months. The snails were assigned to size classes based on shell height. Differences between the size class composition of clutch-bearers and of the general population were statistically compared by means of Pearson’s distance χ²P analysis. Egg clutches were laid on snails of shell height >15 mm (i.e. reproductive-age individuals), with significant selection for the larger size classes (shell height 25–40 mm). While the mechanisms of and reasons behind such behaviour remain unknown, selection of larger adults as egg-carriers may have ecological implications at the population level. KEY WORDS: freshwater gastropods, Lymnaea stagnalis, great pond snail, reproductive behaviour INTRODUCTION Lymnaea stagnalis (Linnaeus, 1758) is a common & CARRIKER 1946, TER MAAT et al. 1989, ELGER & Holarctic freshwater gastropod, inhabiting most of LEMOINE 2005, GROSS & LOMBARDO in press). -

Taxonomic Status of Stagnicola Palustris (O
Folia Malacol. 23(1): 3–18 http://dx.doi.org/10.12657/folmal.023.003 TAXONOMIC STATUS OF STAGNICOLA PALUSTRIS (O. F. MÜLLER, 1774) AND S. TURRICULA (HELD, 1836) (GASTROPODA: PULMONATA: LYMNAEIDAE) IN VIEW OF NEW MOLECULAR AND CHOROLOGICAL DATA Joanna Romana Pieńkowska1, eliza Rybska2, Justyna banasiak1, maRia wesołowska1, andRzeJ lesicki1 1Department of Cell Biology, Institute of Experimental Biology, Faculty of Biology, Adam Mickiewicz University, Umultowska 89, 61-614 Poznań, Poland (e-mail: [email protected], [email protected], [email protected], [email protected]) 2Nature Education and Conservation, Faculty of Biology, Adam Mickiewicz University, Umultowska 89, 61-614 Poznań, Poland ([email protected], [email protected]) abstRact: Analyses of nucleotide sequences of 5’- and 3’- ends of mitochondrial cytochrome oxidase subunit I (5’COI, 3’COI) and fragments of internal transcribed spacer 2 (ITS2) of nuclear rDNA gene confirmed the status of Stagnicola corvus (Gmelin), Lymnaea stagnalis L. and Ladislavella terebra (Westerlund) as separate species. The same results showed that Stagnicola palustris (O. F. Müll.) and S. turricula (Held) could also be treated as separate species, but compared to the aforementioned lymnaeids, the differences in the analysed sequences between them were much smaller, although clearly recognisable. In each case they were also larger than the differences between these molecular features of specimens from different localities of S. palustris or S. turricula. New data on the distribution of S. palustris and S. turricula in Poland showed – in contrast to the earlier reports – that their ranges overlapped. This sympatric distribution together with the small but clearly marked differences in molecular features as well as with differences in the male genitalia between S. -

Gyraulus Laevis in Nederland 87
Kuijper: Gyraulus laevis in Nederland 87 Gyraulus laevis (Mollusca: Planorbidae) in Nederland door W.J. Kuijper Enkele recente waarnemingen van een van onze zeldzaamste planorbiden waren de aanleiding tot het samenstellen van een over- laevis zicht van alle van Nederland bekende vondsten van Gyraulus (Alder, 1838). Tot voor kort waren slechts enkele vindplaatsen van dit dier bekend (Janssen & De Vogel, 1965: 75). Hierbij komt het betrof feit dat het in een aantal gevallen slechts een enkel exemplaar en de soort niet meer teruggevonden kon worden. Het volgende geeft een chronologisch overzicht van de waarnemingen van Gyraulus laevis in Nederland. Voor zover beschikbaar zijn diverse gegevens van de vindplaatsen vermeld. WAARNEMINGEN 1. Koudekerke (bij Middelburg), buitenplaats Vijvervreugd, ± 1890. Fraai vers materiaal: 69 exemplaren in de collectie Schep- de man (Zoölogisch Museum, Amsterdam). Later niet meer vermeld, buitenplaats bestaat niet meer (Kuiper, 1944: 6). Dit materiaalwerd verzameld door Dr. IJ. Keijzer en is vermeld in: Verslag over 1885-1893 van het Zeeuwsch Genootschap der Wetenschappen, blz. 88 (volgens Mevr. Dr. W.S.S. van der Feen - van Benthem Jut- ting). Verder is materiaal van deze vindplaats in de collecties van het Zeeuwsch Museum te Middelburg (2 ex.) en het Natuurhistorisch Museum te Enschede (6 ex.) aanwezig. 2. Warmond (bij Leiden), in de Leede, januari 1916 (Van Ben- them Jutting, 1933: 179). Dit materiaal werd verzameld door P.P. de Koning; in het Rijksmuseum van Natuurlijke Historie te Leiden bevinden zich vier schelpen van deze vindplaats, waarvan twee een doorsnede van ca. 4 mm bereiken. 3. 't Zand (bij Roodeschool), Oostpolder, 1927 (Van Benthem Jutting, 1947: 66). -

Mitochondrial Genome of Bulinus Truncatus (Gastropoda: Lymnaeoidea): Implications for Snail Systematics and Schistosome Epidemiology
Journal Pre-proof Mitochondrial genome of Bulinus truncatus (Gastropoda: Lymnaeoidea): implications for snail systematics and schistosome epidemiology Neil D. Young, Liina Kinkar, Andreas J. Stroehlein, Pasi K. Korhonen, J. Russell Stothard, David Rollinson, Robin B. Gasser PII: S2667-114X(21)00011-X DOI: https://doi.org/10.1016/j.crpvbd.2021.100017 Reference: CRPVBD 100017 To appear in: Current Research in Parasitology and Vector-Borne Diseases Received Date: 21 January 2021 Revised Date: 10 February 2021 Accepted Date: 11 February 2021 Please cite this article as: Young ND, Kinkar L, Stroehlein AJ, Korhonen PK, Stothard JR, Rollinson D, Gasser RB, Mitochondrial genome of Bulinus truncatus (Gastropoda: Lymnaeoidea): implications for snail systematics and schistosome epidemiology, CORTEX, https://doi.org/10.1016/ j.crpvbd.2021.100017. This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. © 2021 The Author(s). Published by Elsevier B.V. Journal Pre-proof Mitochondrial genome of Bulinus truncatus (Gastropoda: Lymnaeoidea): implications for snail systematics and schistosome epidemiology Neil D. Young a,* , Liina Kinkar a, Andreas J. Stroehlein a, Pasi K. Korhonen a, J. -

Invertebrate Responses to Large-Scale Change: Impacts of Eutrophication and Cataclysmic Earthquake Events in a Southern New Zealand Estuary
Invertebrate Responses to Large-Scale Change: Impacts of Eutrophication and Cataclysmic Earthquake Events in a Southern New Zealand Estuary A thesis submitted in partial fulfilment of the requirements for the degree of Doctorate of Philosophy in Ecology at the University of Canterbury, New Zealand Jennifer Erin Skilton 2013 Abstract Abstract Environmental stress and disturbance can affect the structure and functioning of marine ecosystems by altering their physical, chemical and biological features. In estuaries, benthic invertebrate communities play important roles in structuring sediments, influencing primary production and biogeochemical flux, and occupying key food web positions. Stress and disturbance can reduce species diversity, richness and abundance, with ecological theory predicting that biodiversity will be at its lowest soon after a disturbance with assemblages dominated by opportunistic species. The Avon-Heathcote Estuary in Christchurch New Zealand has provided a novel opportunity to examine the effects of stress, in the form of eutrophication, and disturbance, in the form of cataclysmic earthquake events, on the structure and functioning of an estuarine ecosystem. For more than 50 years, large quantities (up to 500,000m3/day) of treated wastewater were released into this estuary but in March 2010 this was diverted to an ocean outfall, thereby reducing the nutrient loading by around 90% to the estuary. This study was therefore initially focussed on the reversal of eutrophication and consequent effects on food web structure in the estuary as it responded to lower nutrients. In 2011, however, Christchurch was struck with a series of large earthquakes that greatly changed the estuary. Massive amounts of liquefied sediments, covering up to 65% of the estuary floor, were forced up from deep below the estuary, the estuary was tilted by up to a 50cm rise on one side and a corresponding drop on the other, and large quantities of raw sewage from broken wastewater infrastructure entered the estuary for up to nine months. -
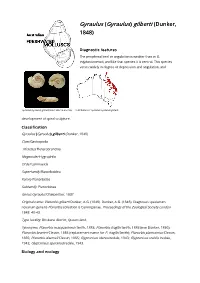
Gyraulus) Gilberti (Dunker, 1848
Gyraulus (Gyraulus) gilberti (Dunker, 1848) Diagnostic features The peripheral keel or angulation is weaker than in G. edgbastonensis, and like that species it is central. This species varies widely in degree of depression and angulation, and Gyraulus (Gyraulus) gilberti (adult size 4.8-5.5 mm) Distribution of Gyraulus (Gyraulus) gilberti. development of spiral sculpture. Classification Gyraulus (Gyraulus) gilberti (Dunker, 1848) Class Gastropoda I nfraclass Heterobranchia Megaorder Hygrophila Order Lymnaeida Superfamily Planorboidea Family Planorbidae Subfamily: Planorbinae Genus Gyraulus Charpentier, 1837 Original name: Planorbis gilberti Dunker, A.G. (1848). Dunker, A.G. (1848). Diagnoses specierum novarum generis Planorbis collection is Cumingianae. Proceedings of the Zoological Society London 1848: 40-43. Type locality: Brisbane district, Queensland. Synonyms: Planorbis macquariensis Smith, 1883; Planorbis fragilis Smith, 1883 (non Dunker, 1850); Planorbis brazieri Clessin, 1885 (replacement name for P. fragilis Smith); Planorbis planissimus Clessin, 1885; Planorbis daemeli Clessin, 1885; Glyptanisus idenusredale, 1943; Glyptanisus stabilis redale, 1943; Glyptanisus speranusredale, 1943. Biology and ecology This species lives in water weeds and other vegetation in ponds, billabongs, swamps and sluggish streams and rivers in tropical and subtropical eastern Australia. Feeds on detritus. Egg mass presumably a jelly strip containing small eggs. Development direct. Brown (2001) described the anatomy of this species. This species is an intermediate host for the stomach fluke Orthocoelium streptocoelium (Boray, 1982; Beesley et al., 1998). Distribution This species occurs throughout eastern Australia, from Cape York to northern New South Wales. Notes G. isingi and/or G.waterhousei may possibly be conspecific with this species (Brown, 2001). Further reading Beesley, P. L., Ross, G. J. -

Anisus Vorticulus (Troschel 1834) (Gastropoda: Planorbidae) in Northeast Germany
JOURNAL OF CONCHOLOGY (2013), VOL.41, NO.3 389 SOME ECOLOGICAL PECULIARITIES OF ANISUS VORTICULUS (TROSCHEL 1834) (GASTROPODA: PLANORBIDAE) IN NORTHEAST GERMANY MICHAEL L. ZETTLER Leibniz Institute for Baltic Sea Research Warnemünde, Seestr. 15, D-18119 Rostock, Germany Abstract During the EU Habitats Directive monitoring between 2008 and 2010 the ecological requirements of the gastropod species Anisus vorticulus (Troschel 1834) were investigated in 24 different waterbodies of northeast Germany. 117 sampling units were analyzed quantitatively. 45 of these units contained living individuals of the target species in abundances between 4 and 616 individuals m-2. More than 25.300 living individuals of accompanying freshwater mollusc species and about 9.400 empty shells were counted and determined to the species level. Altogether 47 species were identified. The benefit of enhanced knowledge on the ecological requirements was gained due to the wide range and high number of sampled habitats with both obviously convenient and inconvenient living conditions for A. vorticulus. In northeast Germany the amphibian zones of sheltered mesotrophic lake shores, swampy (lime) fens and peat holes which are sun exposed and have populations of any Chara species belong to the optimal, continuously and densely colonized biotopes. The cluster analysis emphasized that A. vorticulus was associated with a typical species composition, which can be named as “Anisus-vorticulus-community”. In compliance with that both the frequency of combined occurrence of species and their similarity in relative abundance are important. The following species belong to the “Anisus-vorticulus-community” in northeast Germany: Pisidium obtusale, Pisidium milium, Pisidium pseudosphaerium, Bithynia leachii, Stagnicola palustris, Valvata cristata, Bathyomphalus contortus, Bithynia tentaculata, Anisus vortex, Hippeutis complanatus, Gyraulus crista, Physa fontinalis, Segmentina nitida and Anisus vorticulus. -

Invertebrate Animals (Metazoa: Invertebrata) of the Atanasovsko Lake, Bulgaria
Historia naturalis bulgarica, 22: 45-71, 2015 Invertebrate Animals (Metazoa: Invertebrata) of the Atanasovsko Lake, Bulgaria Zdravko Hubenov, Lyubomir Kenderov, Ivan Pandourski Abstract: The role of the Atanasovsko Lake for storage and protection of the specific faunistic diversity, characteristic of the hyper-saline lakes of the Bulgarian seaside is presented. The fauna of the lake and surrounding waters is reviewed, the taxonomic diversity and some zoogeographical and ecological features of the invertebrates are analyzed. The lake system includes from freshwater to hyper-saline basins with fast changing environment. A total of 6 types, 10 classes, 35 orders, 82 families and 157 species are known from the Atanasovsko Lake and the surrounding basins. They include 56 species (35.7%) marine and marine-brackish forms and 101 species (64.3%) brackish-freshwater, freshwater and terrestrial forms, connected with water. For the first time, 23 species in this study are established (12 marine, 1 brackish and 10 freshwater). The marine and marine- brackish species have 4 types of ranges – Cosmopolitan, Atlantic-Indian, Atlantic-Pacific and Atlantic. The Atlantic (66.1%) and Cosmopolitan (23.2%) ranges that include 80% of the species, predominate. Most of the fauna (over 60%) has an Atlantic-Mediterranean origin and represents an impoverished Atlantic-Mediterranean fauna. The freshwater-brackish, freshwater and terrestrial forms, connected with water, that have been established from the Atanasovsko Lake, have 2 main types of ranges – species, distributed in the Palaearctic and beyond it and species, distributed only in the Palaearctic. The representatives of the first type (52.4%) predomi- nate. They are related to the typical marine coastal habitats, optimal for the development of certain species. -

Freshwater Snail Guide
A Beginner’s Guide to Freshwater Snails of Central Scotland Freshwater snails are a diverse and fascinating group of molluscs found in ponds, lochs, canals, rivers and watery ditches all across the globe. This guide is a simple introduction to some of the most commonly encountered and easily identifiable freshwater snail species in Central Scotland. How do I look for freshwater snails? 1) Choose your spot: anywhere with water – your garden pond, a nearby river, canal or loch – is likely to have snails. 2) Grab a pond net (buy online or make your own by attaching a sieve to the end of an old broom or mop), a white tray or tub and a hand-lens or magnifier. 3) Fill the tray with water from your chosen habitat. Sweep the net around in the water, making sure to get among any vegetation (don’t go too near the bottom or you’ll get a net full of mud), empty your net into the water-filled tray and identify what you have found! Snail shell features Spire Whorls Some snail species have a hard plate called an ‘operculum’ Height which they Mouth (Aperture) use to seal the mouth of the shell when they are inside Height Pointed shell Flat shell Width Width Pond Snails (Lymnaeidae) Variable in size. Mouth always on right-hand side, shells usually long and pointed. Great Pond Snail Common Pond Snail Lymnaea stagnalis Radix balthica Largest pond snail. Common in ponds Fairly rounded and ’fat’. Common in weedy lakes, canals and sometimes slow river still waters. pools. -

A Ma Aeolake in Alacologi N the Moe Cal Analy
Faculty of Sciences Department of Geology and Soil Science Research Unit Palaeontology Academic year 2009‐2010 Changes in surface waters: a malacological analysis of a Late Glacial and early Holocene palaeolake in the Moervaartdepression (Belgium). by Lynn Serbruyns Thesis submitted to obtain the degree of Master in Biology. Promotor: Prof. Dr. Jacques Verniers Co‐promotor: Prof. Dr. Dirk Van Damme Faculty of Sciences Department of Geology and Soil Science Research Unit Palaeontology Academic year 2009‐2010 Changes in surface waters: a malacological analysis of a Late Glacial and early Holocene palaeolake in the Moervaartdepression (Belgium). by Lynn Serbruyns Thesis submitted to obtain the degree of Master in Biology. Promotor: Prof. Dr. Jacques Verniers Co‐promotor: Prof. Dr. Dirk Van Damme Acknowledgements0 First of all, I would like to thank my promoter Prof. Jacques Verniers and Prof. Philippe Crombé for providing me with this interesting subject and for giving me the freedom to further extend the analysis beyond the original boundaries. Thanks to my co-promoter Prof. Dirk Van Damme who I could always contact with questions and who provided me with many articles on the subject. I also want to thank Prof. Keppens for giving me the opportunity to perform the isotope analysis at the VUB, even though technology let us down in the end. I would like to thank Koen Verhoeven for sacrificing part of his office and for aiding me with the sampling from the trench. Thanks to Mona Court-Picon for the numerous ways in which she helped me during the making of this thesis and for the nice talks. -

Speeding up the Snail's Pace Bird
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/93702 Please be advised that this information was generated on 2021-10-07 and may be subject to change. SPEEDING UP THE SNAIL’S PACE Bird-mediated dispersal of aquatic organisms Casper H.A. van Leeuwen Speeding up the snail’s pace Bird-mediated dispersal of aquatic organisms The work in this thesis was conducted at the Netherlands Institute of Ecology (NIOO-KNAW) and Radboud University Nijmegen, cooperating within the Centre for Wetland Ecology. This thesis should be cited as: Van Leeuwen, C.H.A. (2012) Speeding up the snail’s pace: bird-mediated dispersal of aquatic organisms. PhD thesis, Radboud University Nijmegen, Nijmegen, The Netherlands ISBN: 978-90-6464-566-2 Printed by Ponsen & Looijen, Ede, The Netherlands Speeding up the snail’s pace Bird-mediated dispersal of aquatic organisms PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Radboud Universiteit Nijmegen op het gezag van de rector magnificus prof. mr. S.C.J.J. Kortmann, volgens besluit van het College van Decanen in het openbaar te verdedigen op woensdag 27 juni 2012 om 13.00 uur precies door Casper Hendrik Abram van Leeuwen geboren op 18 september 1983 te Odijk Promotoren: Prof. dr. Jan van Groenendael Prof. dr. Marcel Klaassen (Universiteit Utrecht) Copromotor: Dr. Gerard van der Velde Manuscriptcommissie: Prof. dr. Hans de Kroon Dr. Gerhard Cadée (Koninklijk NIOZ) Prof. dr. Edmund Gittenberger (Universiteit Leiden) Prof.