PHOTOSENSITIZING LIST Certain Food/Drugs Do Not Mix with Ultraviolet Light
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
(12) United States Patent (10) Patent No.: US 8,993,581 B2 Perrine Et Al
US00899.3581B2 (12) United States Patent (10) Patent No.: US 8,993,581 B2 Perrine et al. (45) Date of Patent: Mar. 31, 2015 (54) METHODS FOR TREATINGVIRAL (58) Field of Classification Search DSORDERS CPC ... A61K 31/00; A61K 31/166; A61K 31/185: A61K 31/233; A61K 31/522: A61K 38/12: (71) Applicant: Trustees of Boston University, Boston, A61K 38/15: A61K 45/06 MA (US) USPC ........... 514/263.38, 21.1, 557, 565, 575, 617; 424/2011 (72) Inventors: Susan Perrine, Weston, MA (US); Douglas Faller, Weston, MA (US) See application file for complete search history. (73) Assignee: Trustees of Boston University, Boston, (56) References Cited MA (US) U.S. PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this 3,471,513 A 10, 1969 Chinn et al. patent is extended or adjusted under 35 3,904,612 A 9/1975 Nagasawa et al. U.S.C. 154(b) by 0 days. (Continued) (21) Appl. No.: 13/915,092 FOREIGN PATENT DOCUMENTS (22) Filed: Jun. 11, 2013 CA 1209037 A 8, 1986 CA 2303268 A1 4f1995 (65) Prior Publication Data (Continued) US 2014/OO45774 A1 Feb. 13, 2014 OTHER PUBLICATIONS Related U.S. Application Data (63) Continuation of application No. 12/890,042, filed on PCT/US 10/59584 Search Report and Written Opinion mailed Feb. Sep. 24, 2010, now abandoned. 11, 2011. (Continued) (60) Provisional application No. 61/245,529, filed on Sep. 24, 2009, provisional application No. 61/295,663, filed on Jan. 15, 2010. Primary Examiner — Savitha Rao (74) Attorney, Agent, or Firm — Nixon Peabody LLP (51) Int. -

Alzheimer's Disease, a Decade of Treatment: What Have We Learned?
A Critical Look at Medication Dementia: Alzheimer’s Disease Management Issues in Alzheimer’s Disease R.Ron Finley, B.S. Pharm., R.Ph,CGP Lecturer (Emeritus) and Assistant Clinical Professor, UCSF School of Pharmacy Clinical Pharmacist, UCSF Memory and Aging Center- Alzheimer’s Research Center Educational Objectives Disclosures 1. Define the role for cholinesterase inhibitors in the management of Alzheimer’s disease, Lewy Body dementia, Frontal Temporal Lobe dementia. Pfizer Speakers Bureau 2. Name three common side effects of atypical antipsychotic Forest Speakers Bureau drugs. Novartis Speakers Bureau 3. Construct a pharmacological treatment plan for a 77-year-old Rx Consultant Associate Editor patient diagnosed with Alzheimer’s disease and hallucinations. WindChime Consultant 4. Describe the role for antipsychotic, antidepressant, mood HGA HealthCare Consultant stabilizers and benzodiazepines in the management of psychiatric behavior problems related to Alzheimer’s disease. Elder Care Specialist Consultant 5. Cite three potential drug or disease interactions with cholinesterase inhibitors. The Many Faces of Dementia Risk Factors Linked to AD Alzheimer’s Disease Over 65 years of age and increases Vascular: Multi-infarct with age FrontalTemporal Lobe dementia ( FTD) and female Pick’s disease Head injury Lewy Body Dementia Progressive Supranuclear Palsy Factors associated with DM, HTN, CVD Corticobasal Degeneration Genetic: family history, specific Primary Progressive Aphasia chromosome mutations Huntington’s disease -

(12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain Et Al
US 2012O190743A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0190743 A1 Bain et al. (43) Pub. Date: Jul. 26, 2012 (54) COMPOUNDS FOR TREATING DISORDERS Publication Classification OR DISEASES ASSOCATED WITH (51) Int. Cl NEUROKININ 2 RECEPTORACTIVITY A6II 3L/23 (2006.01) (75) Inventors: Jerald Bain, Toronto (CA); Joel CD7C 69/30 (2006.01) Sadavoy, Toronto (CA); Hao Chen, 39t. ii; C Columbia, MD (US); Xiaoyu Shen, ( .01) Columbia, MD (US) A6IPI/00 (2006.01) s A6IP 29/00 (2006.01) (73) Assignee: UNITED PARAGON A6IP II/00 (2006.01) ASSOCIATES INC., Guelph, ON A6IPI3/10 (2006.01) (CA) A6IP 5/00 (2006.01) A6IP 25/00 (2006.01) (21) Appl. No.: 13/394,067 A6IP 25/30 (2006.01) A6IP5/00 (2006.01) (22) PCT Filed: Sep. 7, 2010 A6IP3/00 (2006.01) CI2N 5/071 (2010.01) (86). PCT No.: PCT/US 10/48OO6 CD7C 69/33 (2006.01) S371 (c)(1) (52) U.S. Cl. .......................... 514/552; 554/227; 435/375 (2), (4) Date: Apr. 12, 2012 (57) ABSTRACT Related U.S. Application Data Compounds, pharmaceutical compositions and methods of (60) Provisional application No. 61/240,014, filed on Sep. treating a disorder or disease associated with neurokinin 2 4, 2009. (NK) receptor activity. Patent Application Publication Jul. 26, 2012 Sheet 1 of 12 US 2012/O190743 A1 LU 1750 15OO 1250 OOO 750 500 250 O O 20 3O 40 min SampleName: EM2OO617 Patent Application Publication Jul. 26, 2012 Sheet 2 of 12 US 2012/O190743 A1 kixto CFUgan <tro CFUgan FIG.2 Patent Application Publication Jul. -

Drug-Induced Angle-Closure Glaucoma
10.5005/jp-journals-10008-1100 ArujREVIEW K Khurana ARTICLE et al Drug-induced Angle-Closure Glaucoma Aruj K Khurana, Bhawna Khurana, Ashok K Khurana Regional Institute of Ophthalmology, Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India Correspondence: Ashok K Khurana, Senior Professor, Regional Institute of Ophthalmology, Post Graduate Institute of Medical Sciences, Rohtak, Haryana, India ABSTRACT Drug-induced angle-closure glaucoma is an important entity for the ophthalmologist as well as the general physician as it represents a preventable cause of potential blindness. This brief review highlights the fact that a high index of suspicion, in a susceptible individual followed by confirmation on appropriate imaging modality (UBM, ultrasound or anterior segment OCT) can alleviate the threat to sight and also help to institute appropriate therapy. Keywords: Acute angle closure, Drug-induced glaucoma. INTRODUCTION factors exist for this syndrome.9 Other sulfa-based drugs known Drug-induced angle-closure glaucoma is an important entity to be associated with AACG: Acetazolamide, hydrochloro- 10 for the ophthalmologist as well as the general physician as it thiazide and cotrimoxazole. represents a preventable cause of potential blindness.1 Acute Anticholinergic drugs implicated in the causation of AACG angle-closure glaucoma can develop in a susceptible individual include atropine, homatropine, cyclopentolate and ipratropium by various classes of drugs.2 Practitioners using any of these bromide.10,11 Atropine is often used to treat bradycardia, drugs should be aware of their potential to cause acute angle especially related to general anesthesia. Postoperative AACG closure, such that a patient presenting with signs or symptoms has been reported in patients after general anesthesia for of acute angle closure should be immediately referred to an abdominal, orthopedic, facial and endoscopic surgery.12 3 ophthalmologist. -

Synthetic Drugs
Comprehensive and Confident Identification of Narcotics, Steroids and Pharmaceuticals in Urine David E. Alonso1, Petra Gerhards2, Charles Lyle1 and Joe Binkley1 | 1LECO Corporation, St. Joseph, MI; 2LECO European LSCA Centre, Moenchengladbach, Germany Introduction Experimental Sample A (Traditional Drugs) Sample B (Synthetic Drugs) Monitoring of patients in hospitals and clinics has traditionally relied on Samples Representative Compounds Representative Compounds targeted methods of analysis. These screening methods are not Peak # Name Formula R.T. (s) Area Similarity Mass Delta (mDa) MA (ppm) 1 Creatinine ME C5H9N3O 210 1326229 800 -0.05 -0.43 • Obtained from a collaborating European hospital 3.5e6 3.0e6 Peak # Name Formula R.T. (s) Area Similarity 2 o-Ethynylaniline C8H7N 219 167436 893 0.07 0.63 1 Indole C8H7N 213 2744171 917 comprehensive and result in an incomplete picture of a patient’s 3 2-Methoxy-4-vinylphenol C H O 223 65764 805 0.11 0.73 52 patient monitoring samples 9 10 2 2 Creatinine ME C5H9N3O 216 613318 651 • 2.5e6 4 Nicotine C10H14N2 234 3249121 898 -0.21 -1.29 3 Pyridine, 2-(1-methyl-2-pyrrolidinyl)- C10H14N2 230 24869771 899 activities. Gas chromatography high resolution time-of-flight mass 3.0e6 5 Hordenin C10H15NO 274 949734 775 -0.14 -0.84 4 Parabanic acid, 1-methyl- C4H4N2O3 233 434278 764 Sample preparation 5 Cotinine C10H12N2O 336 27810908 918 • 6 Methylecgonine C10H17NO3 276 104640 835 -0.22 -1.1 spectrometry (GC-HRT) provides a fast and convenient method for 2.0e6 5 6 Caffeine C8H10N4O2 371 3753598 797 2.5e6 7 4-(3-Pyridyl-tetrahydrofuran-2-one C9H9NO2 313 93817 849 -0.11 -0.68 3,4 7 1-methyl-7H-xanthine C6H6N4O2 437 4868196 850 analysis of urine samples. -

Efficacy and Tolerability of Quinacrine Monotherapy and Albendazole Plus Chloroquine Combination Therapy in Nitroimidazole-Refractory Giardiasis: a Tropnet Study
Klinik für Infektiologie & Spitalhygiene Efficacy and tolerability of quinacrine monotherapy and albendazole plus chloroquine combination therapy in nitroimidazole-refractory giardiasis: a TropNet study Andreas Neumayr, Mirjam Schunk, Caroline Theunissen, Marjan Van Esbroeck, Matthieu Mechain, Manuel Jesús Soriano Pérez, Kristine Mørch, Peter Sothmann, Esther Künzli, Camilla Rothe, Emmanuel Bottieau Journal Club 01.03.21 Andreas Neumayr Background on giardia treatment: • 1st-line treatment: 5-nitroimidazoles: metronidazole (1957), tinidazole, ornidazole, secnidazole • cure rate of 5NIs in 1st-line treatment: ~90% • in the last decade, an increase of 5NI-refractory giardia cases has been observed in travel medicine clinics across Europe: Hospital for Tropical Diseases, London: 2008: 15% --> 2013: 40% 70% of 5NI-refractory cases imported from India • 2nd-line treatment: effectiveness of a 2nd round with a 5NI: ~17% alternative drugs: albendazole, mebendazole, nitazoxanide, quinacrine, furazolidone, chloroquine, paromomycin 2012 TropNet member survey: 53 centres use 39 different treatment regimens, consisting of 7 different drugs in mono- or combination-therapy in various dosages and durations JC 01.03.21 Nabarro LE et al. Clin Microbiol Infect. 2015;21:791-6. • by 2013, there were only 13 reports of 2nd-line therapy for giardiasis (8 case series, 5 individual case reports): n=110 Cure rates Albendazole 6/32 18.7% Paromomycin 5/17 29.4% Nitazoxanide 2/5 40.0% Albendazole + 5-NI 42/53 79.2% Quinacrine 19/21 90.5% Quinacrine + 5-NI 14/14 100% Quinacrine + Paromomycin 2/2 100% • 2013: TropNet "GiardiaREF" study kick-off: Study on efficacy and tolerability of two 2nd-line regimens in nitroimidazole-refractory giardiasis: Quinacrine JC 01.03.21 Meltzer E et al. -

Dilantin (Phenytoin Sodium) Extended Oral Capsule Three Times Daily and the Dosage Then Adjusted to Suit Individual Requirements
Dilantin® (Phenytoin Sodium) 100 mg Extended Oral Capsule DESCRIPTION Phenytoin sodium is an antiepileptic drug. Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione, having the following structural formula: Each Dilantin— 100 mg Extended Oral Capsule—contains 100 mg phenytoin sodium. Also contains lactose monohydrate, NF; confectioner’s sugar, NF; talc, USP; and magnesium stearate, NF. The capsule body contains titanium dioxide, USP and gelatin, NF. The capsule cap contains FD&C red No. 28; FD&C yellow No. 6; and gelatin NF. Product in vivo performance is characterized by a slow and extended rate of absorption with peak blood concentrations expected in 4 to 12 hours as contrasted to Prompt Phenytoin Sodium Capsules, USP with a rapid rate of absorption with peak blood concentration expected in 1½ to 3 hours. CLINICAL PHARMACOLOGY Phenytoin is an antiepileptic drug which can be used in the treatment of epilepsy. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of posttetanic potentiation at synapses. Loss of posttetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Phenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of tonic-clonic (grand mal) seizures. The plasma half-life in man after oral administration of phenytoin averages 22 hours, with a range of 7 to 42 hours. -
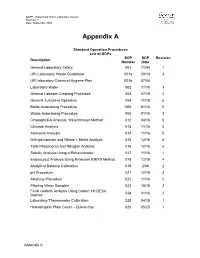
Lab Standard Operating Procedures (Sops)
QAPP –Watershed Watch Laboratory Assays Revision: 7 Date: September 2020 Appendix A Standard Operation Procedures List of SOPs SOP SOP Revision Description Number Date General Laboratory Safety 001 11/04 1 URI Laboratory Waste Guidebook 001a 09/13 3 URI laboratory Chemical Hygiene Plan 001b 07/04 Laboratory Water 002 11/16 3 General Labware Cleaning Procedure 003 07/19 4 General Autoclave Operation 004 10/18 6 Bottle Autoclaving Procedure 005 01/16 5 Waste Autoclaving Procedure 006 01/16 3 Chlorophyll-A Analysis, Welschmeyer Method 012 04/18 6 Chloride Analysis 013 11/16 5 Ammonia Analysis 014 11/16 5 Orthophosphate and Nitrate + Nitrite Analysis 015 12/16 5 Total Phosphorus and Nitrogen Analysis 016 12/16 5 Salinity Analysis Using a Refractometer 017 11/16 1 Enterococci Analysis Using Enterolert IDEXX Method 018 12/19 4 Analytical Balance Calibration 019 2/06 2 pH Procedure 021 12/19 3 Alkalinity Procedure 022 11/16 2 Filtering Water Samples 023 10/18 2 Fecal coliform Analysis Using Colilert 18 IDEXX 024 01/16 2 Method Laboratory Thermometer Calibration 025 04/19 1 Heterotrophic Plate Count – Quanti-tray 026 05/20 1 Appendix A Standard Operating Procedure 001 Date: 11/04 General Laboratory Safety Revision: 1 Author: Linda Green University of Rhode Island Watershed Watch 1.0 PURPOSE AND DESCRIPTION LAB SAFETY IS EVERYBODY’S JOB! Please be sure to familiarize yourself with these general procedures, as well as the specific handling requirements included in the Standard Operating Procedure (SOP) for each analysis/process. Further general information regarding University of Rhode Island standards for health and safety are found in SOP 001a – University Safety and Waste Handling Document. -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Non-Steroidal Drug-Induced Glaucoma MR Razeghinejad Et Al 972
Eye (2011) 25, 971–980 & 2011 Macmillan Publishers Limited All rights reserved 0950-222X/11 www.nature.com/eye 1,2 1 1 Non-steroidal drug- MR Razeghinejad , MJ Pro and LJ Katz REVIEW induced glaucoma Abstract vision. The majority of drugs listed as contraindicated in glaucoma are concerned with Numerous systemically used drugs are CAG. These medications may incite an attack in involved in drug-induced glaucoma. Most those individuals with narrow iridocorneal reported cases of non-steroidal drug-induced angle.3 At least one-third of acute closed-angle glaucoma are closed-angle glaucoma (CAG). glaucoma (ACAG) cases are related to an Indeed, many routinely used drugs that have over-the-counter or prescription drug.1 Prevalence sympathomimetic or parasympatholytic of narrow angles in whites from the Framingham properties can cause pupillary block CAG in study was 3.8%. Narrow angles are more individuals with narrow iridocorneal angle. The resulting acute glaucoma occurs much common in the Asian population. A study of a more commonly unilaterally and only rarely Vietnamese population estimated a prevalence 4 bilaterally. CAG secondary to sulfa drugs is a of occludable angles at 8.5%. The reported bilateral non-pupillary block type and is due prevalence of elevated IOP months to years to forward movement of iris–lens diaphragm, after controlling ACAG with laser iridotomy 5,6 which occurs in individuals with narrow or ranges from 24 to 72%. Additionally, a open iridocorneal angle. A few agents, significant decrease in retinal nerve fiber layer including antineoplastics, may induce thickness and an increase in the cup/disc ratio open-angle glaucoma. -

Jos Journal 2
POST-OPERATIVE AUDIT OF G6PD-DEFICIENT MALE CHILDREN WITH OBSTRUCTIVE ADENOTONSILLAR ENLARGEMENT AT UNIVERSITY COLLEGE HOSPITAL, IBADAN, NIGERIA. John EN1, Totyen EL1, Jacob N2, Nwaorgu OGB1 1 .Department of ENT/Head and Neck Surgery, University College Hospital, Ibadan, Nigeria 2. Department of paediatrics, University College Hospital, Ibadan, Nigeria All correspondences and request for reprint to Dr John EN, Department of ENT/Head and Neck Surgery, University College Hospital, Ibadan, Nigeria Email: [email protected] Telephone: +2348036240109 Abstract Background: G6PD deficiency ranks among the commonest hereditary enzyme deficiency worldwide and notable as a predisposing condition to haemolyticcrises. The fear of possible untoward effects is often expressed by parents of G6PD deficient male children scheduled for surgery after obtaining an informed and understood consent. The parental perception of obstructive adenotonsillar enlargement in this condition was also appraised. Methods: A retrospective chart review of all G6PD deficient male children between ages 1 to 7years who had adenotonsillectomy over a 3year period at University college Hospital, Ibadan, Nigeria. Results: The patients comprised of 22 G6PD deficient male children diagnosed shortly after birth upon development of neonatal jaundice. Fifteen(68.2%) and 6(27.3%) of the patients subsequently developed episodes of drug- induced haemolysis and non-haemolytic drug reactions prior to undergoing adenotonsillectomy by the otolaryngologists. None of the patients was observed to develop haemolytic crises up to 2weeks post-adenotonsillectomy. From the parental perception and responses in the follow-up period,all 22(100%) patient had resolution of noisy breathing, 20(91%) had improvement of snoring and apnoeic spells. Only 15 (68%) were reported to stop mouth-breathing. -

THE SPECIFICITY of DRUG BINDING SITES on HUMAN SERUM ALBUMIN Ingvar Sjòholm
THE SPECIFICITY OF DRUG BINDING SITES ON HUMAN SERUM ALBUMIN Ingvar Sjòholm Today, it is well established that the binding of drugs in serum will strongly influence the pharmacokinetic parameters of a drug, such as its distribution volume and clearance. It is also evident that the binding of the drug—in serum and elsewhere in the tissues—will have an influence on the duration and intensity of the pharmacological effect. Several excellent papers and reviews have dealt with these issues in recent years.1"** It is obvious that albumin, being the most abundant protein species in the extracellular fluids, is the most im- portant drug-binding protein, although other proteins can play a pharmacokinetic role. Thus, e.g., orosomucoid (aj-acid glyco- protein) can bind some basic and neutral drugs ,9 and lipoproteins some highly hydrophobic drugs.10 The primary structure of human serum albumin (HSA) is now known.1:L'^2 However, all efforts to study the three-dimensional structure by x-ray spectroscopy have hitherto failed, and a detailed knowledge of the mechanisms involved in the binding of drugs or endogenous compounds is still missing. The broad binding specificity of HSA is remarkable. Several compounds of widely different struc- ture can be bound with high affinity—e.g., fatty acids, bilirubin, tryptophan, as well as many drugs. It is also striking that different reports from quantitative studies on the binding of different com- pounds have shown varying results, which cannot be solely explained by technical problems or different experimental conditions. All avail- able information indicates that HSA is a highly "flexible" and "adapt- able" molecule, the structure of which can be strongly influenced by different "modulating" substances.