Propagation of Comptonia Peregrina L. from Stem Cuttings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marilandica, Summer/Fall 2002
MARILANDICA Journal of the Maryland Native Plant Society Vol. 10, No. 2 Summer/Fall 2002 ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ Marilandica Journal of the Maryland Native Plant Society The Maryland Native Volume 10, Number 2 Summer/Fall 2002 Plant Society ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ (MNPS) is a nonprofit organization that uses education, research, and Table of Contents community service to increase the awareness and appreciation of Native Woody Flora of Montgomery County native plants and their habitats, By John Mills Parrish leading to their conservation and Page 3 restoration. Membership is open to ~ all who are interested in Maryland’s MNPS Field Botany Updates native plants and their habitats, preserving Maryland’s natural By Rod Simmons, Cris Fleming, John Parrish, and Jake Hughes heritage, increasing their knowledge Page 8 of native plants, and helping to ~ further the Society’s mission. In Search of Another Orchid Species By Joseph F. Metzger, Jr. MNPS sponsors monthly meetings, Page 11 workshops, field trips, and an ~ annual fall conference. Just Boil the Seeds By James MacDonald Page 13 Maryland Native Plant Society ~ P.O. Box 4877 MNPS Contacts Silver Spring, MD 20914 www.mdflora.org Page 15 ~ Some Varieties of Andropogon virginicus and MNPS Executive Officers: Andropogon scoparius By M.L. Fernald, Rhodora, Vol. 37, 1935 Karyn Molines-President Page 16 Louis Aronica-Vice President Marc Imlay-Vice President Roderick Simmons-Vice President Jane Osburn-Secretary Jean Cantwell-Treasurer MNPS Board Of Directors: Carole Bergmann Blaine Eckberg Cris Fleming Jake Hughes Carol Jelich Dwight Johnson James MacDonald Joe Metzger, Jr. Lespedeza repens John Parrish Mary Pat Rowan Submissions for Marilandica are welcomed. Word documents are preferred but Louisa Thompson not necessary. -

Coastal Landscaping in Massachusetts Plant List
Coastal Landscaping in Massachusetts Plant List This PDF document provides additional information to supplement the Massachusetts Office of Coastal Zone Management (CZM) Coastal Landscaping website. The plants listed below are good choices for the rugged coastal conditions of Massachusetts. The Coastal Beach Plant List, Coastal Dune Plant List, and Coastal Bank Plant List give recommended species for each specified location (some species overlap because they thrive in various conditions). Photos and descriptions of selected species can be found on the following pages: • Grasses and Perennials • Shrubs and Groundcovers • Trees CZM recommends using native plants wherever possible. The vast majority of the plants listed below are native (which, for purposes of this fact sheet, means they occur naturally in eastern Massachusetts). Certain non-native species with specific coastal landscaping advantages that are not known to be invasive have also been listed. These plants are labeled “not native,” and their state or country of origin is provided. (See definitions for native plant species and non-native plant species at the end of this fact sheet.) Coastal Beach Plant List Plant List for Sheltered Intertidal Areas Sheltered intertidal areas (between the low-tide and high-tide line) of beach, marsh, and even rocky environments are home to particular plant species that can tolerate extreme fluctuations in water, salinity, and temperature. The following plants are appropriate for these conditions along the Massachusetts coast. Black Grass (Juncus gerardii) native Marsh Elder (Iva frutescens) native Saltmarsh Cordgrass (Spartina alterniflora) native Saltmeadow Cordgrass (Spartina patens) native Sea Lavender (Limonium carolinianum or nashii) native Spike Grass (Distichlis spicata) native Switchgrass (Panicum virgatum) native Plant List for a Dry Beach Dry beach areas are home to plants that can tolerate wind, wind-blown sand, salt spray, and regular interaction with waves and flood waters. -

Appendix 2: Plant Lists
Appendix 2: Plant Lists Master List and Section Lists Mahlon Dickerson Reservation Botanical Survey and Stewardship Assessment Wild Ridge Plants, LLC 2015 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Acalypha rhomboidea Native 1 Forb 9 Acer palmatum Invasive 0 Tree 1 Acer pensylvanicum Native 7 Tree 2 Acer platanoides Invasive 0 Tree 4 Acer rubrum Native 3 Tree 27 Acer saccharum Native 5 Tree 24 Achillea millefolium Native 0 Forb 18 Acorus calamus Alien 0 Forb 1 Actaea pachypoda Native 5 Forb 10 Adiantum pedatum Native 7 Fern 7 Ageratina altissima v. altissima Native 3 Forb 23 Agrimonia gryposepala Native 4 Forb 4 Agrostis canina Alien 0 Graminoid 2 Agrostis gigantea Alien 0 Graminoid 8 Agrostis hyemalis Native 2 Graminoid 3 Agrostis perennans Native 5 Graminoid 18 Agrostis stolonifera Invasive 0 Graminoid 3 Ailanthus altissima Invasive 0 Tree 8 Ajuga reptans Invasive 0 Forb 3 Alisma subcordatum Native 3 Forb 3 Alliaria petiolata Invasive 0 Forb 17 Allium tricoccum Native 8 Forb 3 Allium vineale Alien 0 Forb 2 Alnus incana ssp rugosa Native 6 Shrub 5 Alnus serrulata Native 4 Shrub 3 Ambrosia artemisiifolia Native 0 Forb 14 Amelanchier arborea Native 7 Tree 26 Amphicarpaea bracteata Native 4 Vine, herbaceous 18 2015 MASTER PLANT LIST MAHLON DICKERSON RESERVATION SCIENTIFIC NAME NATIVENESS S-RANK CC PLANT HABIT # OF SECTIONS Anagallis arvensis Alien 0 Forb 4 Anaphalis margaritacea Native 2 Forb 3 Andropogon gerardii Native 4 Graminoid 1 Andropogon virginicus Native 2 Graminoid 1 Anemone americana Native 9 Forb 6 Anemone quinquefolia Native 7 Forb 13 Anemone virginiana Native 4 Forb 5 Antennaria neglecta Native 2 Forb 2 Antennaria neodioica ssp. -

Plant Fact Sheet
Plant Fact Sheet hair-covered leaves are 2-5 inches long and taper at SWEETFERN each end. There is an occasional compound leaf form variation. The leaf blades are deeply cut into 20 Comptonia peregrina L. or more rounded lobes, dark green above, paler and Plant Symbol = COPE80 hair-covered beneath and on the midrib and margin above. Resinous glands cover both surfaces. Leaves Contributed by: USDA NRCS Plant Materials are very aromatic when crushed. The flowers are Program small, inconspicuous catkins that bloom during April and May. Flowers of one or both sexes can be produced on an individual plant. The male catkins are rather long and cylindrical; the female catkins are short and rounded. In winter, the male catkins are prominent and erect. The female catkins become bur-like at maturity and are 1/2 inch in diameter. The seeds are nutlets that mature in August and become available in September and October. About four seeds are found in each fruit. Each seed is about 1/4 inch long, olive brown in color, and shiny. Adaptation and Distribution USDA NRCS National Plant Materials Center Sweetfern does especially well in open, sterile, sandy Beltsville, MD soils of woodlands, clearings, and pastures. It prefers acidic soils over limestone soils. Alternate Names Sweetfern is distributed throughout northeastern Comptonia peregrina (L.) Coult. var. aspleniifolia United States. For a current distribution map, please (L.) Fern., Myrica aspleniifolia L., Myrica peregrina consult the Plant Profile page for this species on the (L.) Kuntze PLANTS Website. Uses Establishment This nitrogen-fixing plant is used primarily as a Some nurseries offer wild collected clumps, but it is ground cover for erosion control and species diversity best established using nursery-grown, containerized in sterile, sandy soils. -

The Ecology of Sweet Fern
Bulldozers and Bacteria: The Ecology of Sweet Fern Peter Del Tredici Comptonia peregrina, a common roadside plant in eastern North America, provides a case study both of how nature copes with disturbance to the land and of just how convoluted the study of this process can be. Sweet fern, Comptonia peregrina, is a shrubby soils. The first is its use of nitrogen gas from member of the Myricaceae, or bayberry family. the atmosphere to produce mtrates-a feat it Its common name is derived from the pleasing accomplishes by forming root nodules in symbi- fragrance that its tiny, resin-filled, glandular otic association with nitrogen-fixing bacteria. hairs give off when crushed or rubbed, and from The second is an ability to propagate itself veg- its coarsely lobed, somewhat fern-like leaves. etatively by means of long, thick roots that run Comptoma, a distinctly unprepossessing plant, an inch or so beneath the soil surface. These has a natural range that covers a large portion of shallow roots form numerous buds in the fall eastern North America. Forming a rough tri- that grow into shoots the following spring. angle, the eastern flank of this range extends Under the right conditions, Comptonia behaves from Prince Edward Island and Nova Scotia as a shrubby groundcover, spreading over large south into the mountains of north Georgia; the areas by means of these root suckers. western edge reaches from the southern Appala- chians north through Tennessee and Minnesota Historical Considerations all the way to central Manitoba; and the north- Sweet fern’s distinctive form and pungent odor ern edge runs from the Canadian plains through made a strong impression on the early European central Ontario and Quebec to the Atlantic settlers of North America. -

MILKWEED: a SPECIAL STORY Can You Guess Why? NRCS PLANTS Database PLANTS NRCS
NATIVE PLANT GARDEN Wild columbine (Aquilegia canadensis) is a good source of The species of native plants you see here nectar for hummingbirds. MILKWEED: A SPECIAL STORY Can you guess why? NRCS PLANTS Database PLANTS NRCS have been growing in this region since - before European settlers arrived. Native Lobos Lobos USDA @ plants are important habitat for wildlife, - providing food and shelter. Villa Jane NRCS PLANTS Database PLANTS NRCS - @ USDA @ One of autumn’s showiest flowers, New Green, USFS Green, England aster (Symphyotrichum novae- Mohlenbrock Melissa Melissa angliae) attracts moths and butterflies, and Robert H. H. Robert is an important source of nectar for bees. Thomas G. Barnes, University of Kentucky University Barnes, G. Thomas NRCS PLANTS Database PLANTS NRCS - @ USDA @ Allain http://www.hort.uconn.edu Larry Larry An indicator of poor soil, sweetfern (Comptonia peregrina) Grey-headed coneflower (Ratibida pinnata) attracts is not really a fern at all; look for its woody stems. Its butterflies and is a food source for birds in the fall. USFS Green, leaves are very aromatic when crushed. Melissa Melissa Common milkweed (Asclepias syriaca) is critical to the life cycle of monarch Look for wild geranium butterflies. Monarchs eat the flower nectar (Geranium and lay their eggs only on milkweed. The maculatum) larvae are not affected by the poison they flowers in late spring. absorb from the leaves; instead they become NRCS PLANTS Database PLANTS NRCS - distasteful to potential predators. Monarchs complete a multi-generational migration G.A. Cooper @ USDA @ Cooper G.A. each year between Mexico and the northern The attractive flowers and colorful berries of bearberry (Arctostaphylos uva-ursi), also called kinnikinnick, make it a U.S. -

An Analysis of the Vascular Flora of Annapolis Heathlands, Nova Scotia
12_05055_flora.qxd 11/1/07 11:11 AM Page 351 An Analysis of the Vascular Flora of Annapolis Heathlands, Nova Scotia S. CARBYN1, P. M. CATLING2, S. P. VANDER KLOET3, and S. BASQUILL4 1Agriculture and Agri-Food Canada, Environmental Health, Biodiversity, 32 Main Street, Kentville, Nova Scotia B4N 1J5 2 Agriculture and AgriFood Canada, Environmental Health, Biodiversity, Saunders Bldg., Central Experimental Farm, Ottawa, Ontario K1A 0C6 Canada; e-mail: [email protected] 3Department of Biology, Acadia University, Wolfville, Nova Scotia B4P 2R6 Canada 4Atlantic Canada Conservation Data Centre, PO Box 6416, Sackville, New Brunswick E4L 1G6 Canada; e-mail: sbasquill@ mta.ca Carbyn, S., P. M. Catling, S. P. Vander Kloet, and S. Basquill. 2006. An analysis of the vascular flora of Annapolis Heath- lands, Nova Scotia. Canadian Field-Naturalist 120(3): 351–362. A description and analysis of the vascular plant composition of heathlands in the Annapolis valley were undertaken to pro- vide a basis for biodiversity preservation within a system of protected sites. Species presence and abundance were recorded at 23 remnant sites identified using topographic maps, air photos, and Nova Scotia Department of Natural Resources records. A total of 126 species was recorded, of which 94 were native and 31 introduced. The Annapolis heathland remnants are strongly dominated by Corema conradii with Comptonia peregrina, Vaccinium angustifolium and Pteridium aquilinum var. latiusculum. A number of species, including Solidago bicolor, Carex tonsa var. rugosperma, Dichanthelium depauperatum, Lechea intermedia, Melampyrum lineare, and Rubus hispidus, were characteristic of heathland remnants, although they usu- ally contributed little to the total cover. -

Myrica Faya: Review of the Biology, Ecology, Distribution, and Control, Including an Annotated Bibliography Candace J
COOPERATIVE NATIONAL PARK RESOURCES STUDIES UNIT UNIWRSITY OF HAWAI'I AT MANOA Department of Botany 3190 Maile Way Honolulu, Hawai'i 96822 (808) 956-821 8 Technical Report 94 Myrica faya: Review of the Biology, Ecology, Distribution, and Control, Including an Annotated Bibliography Candace J. Lutzow-Felling, Donald E. Gardner, George P. Markin, Clifford W. Smith UNIVERSITY OF HAWAI'I AT MANOA NATIONAL PARK SERVICE Cooperative Agreement CA 8037-2-0001 April 1995 TABLE OF CONTENTS ... LIST OF FIGURES ...................................................................................................... 111 ABSTRACT ...................................................................................................................... v INTRODUCTION ............................................................................................................. 1 DESCRIPTIVE BIOLOGY ............................................................................................. 2 Systematics .................................... ............................................................................ 2 Anatomy ..................................................................................................................... 4 Growth Form ................................................................................................................ 4 Reproductive Structures ...............................................................................................5 Inflorescence ...................... ... ..........................................................................5 -
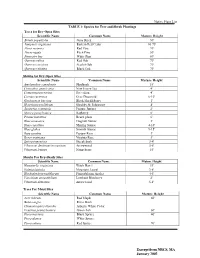
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees For
Native Plant List TABLE 1: Species for Tree and Shrub Plantings Trees for Dry-Open Sites Scientific Name Common Name Mature Height Betula populifolia Gray Birch 30' Juniperis virginiana Eastern Red Cedar 10-75' Pinus resinosa Red Pine 70' Pin us rigida Pitch Pine 50' Pinus stro bus White Pine 80' Quercus rubra Red Oak 70' Quercus coccinea Scarlet Oak 70' Quercus velutina Black Oak 70' Shrubs for Dry-Open Sites Scientific Name 'Common Name Mature Height Amelanchier canadensis Shadbush 15' Ceanothus americanus New Jersey Tea 4' Comptonia peregrina Sweetfern 4' Cornus racemosa Gray Dogwood 6-10' Gaylussacia baccata Black Huckleberry l' Hypericum prolificum Shrubby St. Johnswort 4' Juniperus communis Pasture Juniper 2' Myrica pensylvanica Bayberry 6' Prunus maritima Beach plum 6' Rhus aromatica Fragrant Sumac 3' Rhus copallina Shining Sumac 4-10' Rhus glabra Smooth Sumac 9-15' Rosa carolina Pasture Rose 3' Rosa virginiana Virginia Rose 3' Spirea tomentosa Steeplebush 3-4' Viburnum dentatum/recognitum Arrowwood 5-8' Viburnum lentago Nannyberry 15' Shrubs For Dry-Shady Sites Scientific Name Common Name Mature Height Hamamelis wrginiana Witch Hazel 15' Kalmia latifolia Mountain Laurel 3-8' Rhododendron nudiflorum Pinxterbloom Azalea 4-6' Vaccinium angustifolium Lowbush Blueberry 2' Viburnum dentatum Arrowwood 5-8' Trees For Moist Sites Scientific Name Common Name Mature Height Acer rubrum Red Maple 60' Betula nigra River Birch Chamaecyparis thyoides Atlantic White Cedar Fraxinus pennsylvanica Green Ash 60' Picea mariana Black Spruce 40' Picea -

APPROVED PLANT LIST for JURISDICTIONAL PROJECTS
CARLISLE CONSERVATION COMISSION - APPROVED PLANT LIST for JURISDICTIONAL PROJECTS UNDER FORESTED BANKS & WATER WETLAND DRIER WETLANDS WET, SUNNY WATER'S (>1' INDICATOR TYPE BOTANICAL NAME COMMON NAME UPLAND & SHADE MEADOWS EDGE DEEP) STATUS Deciduous Trees Acer rubrum Red Maple x x x x FAC Acer saccharinum Silver Maple x x x x FACW Benthamidia (Cornus) florida Flowering Dogwood x x x FACU Betula nigra River Birch x x x x FACW Betula papyrifera Paper Birch x FACU Hamamelis virginiana Witch-Hazel x x FAC- Nyssa sylvatica Sweet Gum x x x x FAC Quercus alba White Oak x x x FACU Quercus rubra Red Oak x x x FACU- Evergreen Trees Juniperus virginiana Eastern Red Cedar x x UPL Pinus strobus White Pine x x x FACU Tsuga canadensis Eastern Hemlock x x FACU Evergreen Shrubs Juniperus horizontalis Prostrate Juniper x x FACU Kalmia latifolia Mountain Laurel x x x FACU Shrubs Amelanchier canadensis ShadBush x x x FAC Cephalanthus occidentalis Buttonbush x x x x x OBL Clethra alnifolia Sweet Pepper Bush x x x x x FAC+ Comptonia peregrina Sweet Fern x x UPL Corylus americana American Hazelnut x x x x FACU- Ilex verticillata Winterberry Holly x x x x FACW+ Lindera benzoin Spice Bush x x x FACW- Photinia (Aronia) arbutifolia Red Chokeberry x x FACW Photinia (Aronia) melanocarpa Black Chokeberry x x FAC Rhododendron viscosum Swamp Azalea x x x OBL Swida (Cornus) racemosa Grey Dogwood x x x x FAC Swida (Cornus) sericea Red Osier Dogwood x x x x FACW+ Vaccinium angustifolium Low Bush Blueberry x x x FACU- Vaccinium corymbosum High Bush Blueberry x x x x FACW -

Calvert County Native Plant List
February 2011 CALVERT COUNTY NATIVE PLANT LIST Canopy Trees (Generally > 35 ft. tall at maturity) Planting Stock: 2-in. caliper in size spaced 20-40 ft. on center Common Name Species Notes Box Elder Acer negundo Red Maple Acer rubrum Silver Maple Acer saccharinum River Birch Betula nigra Bitternut Hickory Carya cordiformis Pignut Hickory Carya glabra Shagbark Hickory Carya ovata Mockernut Hickory Carya tomentosa Common Hackberry Celtis occidentalis Atlantic White Cedar Chamaecyparis thyoides Evergreen Common Persimmon Diospyros virginiana American Beech Fagus grandifolia Black Walnut Juglans nigra Eastern Red Cedar Juniperus virginiana Evergreen Sweet Gum Liquidambar styraciflua Tulip Poplar Liriodendron tulipifera Red Mulberry Morus rubra Black Gum Nyssa sylvatica Salt Tolerant Shortleaf Pine Pinus echinata Evergreen Pitch Pine Pinus rigida Evergreen; Salt Tolerant Pond Pine Pinus serotina Evergreen Loblolly Pine Pinus taeda Evergreen Virginia Pine Pinus virgiana Evergreen American Sycamore Platanus occidentalis Black Cherry Prunus serotina Salt Tolerant White Oak Quercus alba Salt Tolerant Swamp White Oak Quercus bicolor Salt Tolerant Scarlet Oak Quercus coccinea Salt Tolerant Southern Red Oak Quercus falcata Blackjack Oak Quercus marilandica Swamp Chestnut Oak Quercus michauxii Chinquapin Oak Quercus muehlenbergii Water Oak Quercus nigra Pin Oak Quercus palustris Salt Tolerant Willow Oak Quercus phellos Chestnut Oak Quercus prinus Northern Red Oak Quercus rubra Salt Tolerant Post Oak Quercus stellata Salt Tolerant Black Oak Quercus velutina Salt Tolerant Black Locust Robinia pseudoacacia Bald Cypress Taxodium distichum Salt Tolerant American Basswood Tilia americana American Elm Ulmus americana 1 February 2011 CAL VERT COUNTY NATIVE PLANTS LIST (cont.) Understory Trees (Generally < 35 ft. tall at maturity) Planting Stock: 1 to 2-in. -
Native Plants of Maryland: What, When and Where
Home and Garden Mimeo HG#120 3/2005 Native Plants of Maryland: What, When and Where Eupatorium Cercis fistulosum canadensis Monarda didyma Rhododendron periclymenoides Tradescantia virginiana Tiarella cordifolia Rudbeckia hirta Lobelia cardinalis TABLE OF CONTENTS What are Native Plants ....................................... 2 Plant listings by preferred conditions .......... 15-20 Physiographic Map of Maryland ........................ 2 Plant Common Name Index ......................... 20-22 Invasive Non Natives .......................................... 3 References ........................................................ 23 Plant listing by type and preferences ............ 4-14 Glossary ............................................................ 23 Native Plants for Maryland INTRODUCTION WHAT ARE GROWTH CONDITIONS FOR NATIVE PLANTS? This guide is intended to help in the selection of native plants for habitat restoration, Maryland is host to a wide variety of native plants. This is due to the diversity of geo- critical area buffer management and natural landscaping projects. All of these plants graphical and climatic conditions. The state is divided into three physiographic regions are native to Maryland. Each section lists plants in alphabetical order by their Latin coastal, piedmont and mountain. You may use the map below to determine your region. names. Common names are included and are cross-referenced in the index. Growth conditions and plant characteristics are also included. State of Maryland Physiographic Regions WHAT ARE NATIVE PLANTS? A native plant is a species that originates or occurs naturally in a particular region. As our local habitat is disturbed by development, non-native and invasive plants change the character of our landscapes. Although many naturalized but introduced plants occur in most regions, the native plants listed are species that existed in Maryland when the European settlers arrived, or they are cultivars of these species.