Recruitment Constraints in Singapore's Fluted Giant Clam
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2 Parks & Waterbodies Plan
SG1 Parks & Waterbodies Plan AND IDENTITY PLAN S UBJECT G ROUP R EPORT O N PARKS & WATERBODIES PLAN AND R USTIC C OAST November 2002 SG1 SG1 S UBJECT G ROUP R EPORT O N PARKS & WATERBODIES PLAN AND R USTIC C OAST November 2002 SG1 SG1 SG1 i 1 INTRODUCTION 1.1 The Parks & Waterbodies Plan and the Identity Plan present ideas and possibilities on how we can enhance our living environment by making the most of our natural assets like the greenery and waterbodies and by retaining places with local identity and history. The two plans were put to public consultation from 23 July 2002 to 22 October 2002. More than 35,000 visited the exhibition, and feedback was received from about 3,600 individuals. Appointment of Subject Groups 1.2 3 Subject Groups (SGs) were appointed by Minister of National Development, Mr Mah Bow Tan as part of the public consultation exercise to study proposals under the following areas: a. Subject Group 1: Parks and Waterbodies Plan and the Rustic Coast b. Subject Group 2: Urban Villages and Southern Ridges & Hillside Villages c. Subject Group 3: Old World Charm 1.3 The SG members, comprising professionals, representatives from interest groups and lay people were tasked to study the various proposals for the 2 plans, conduct dialogue sessions with stakeholders and consider public feedback, before making their recommendations to URA on the proposals. Following from the public consultation exercise, URA will finalise the proposals and incorporate the major land use changes and ideas into the Master Plan 2003. -

Country Report Singapore
Country Report Singapore Natural Disaster Risk Assessment and Area Business Continuity Plan Formulation for Industrial Agglomerated Areas in the ASEAN Region March 2015 AHA CENTRE Japan International Cooperation Agency OYO International Corporation Mitsubishi Research Institute, Inc. CTI Engineering International Co., Ltd. Overview of the Country Basic Information of Singapore 1), 2), 3) National Flag Country Name Long form : Republic of Singapore Short form : Singapore Capital Singapore (city-state) Area (km2) Total: 716 Land: 700 Inland Water: 16 Population 5,399,200 Population density(people/ km2 of land area) 7,713 Population growth (annual %) 1.6 Urban population (% of total) 100 Languages Malay (National/Official language), English, Chinese, Tamil (Official languages) Ethnic Groups Chinese 74%, Malay 13%, Indian 9%, Others 3% Religions Buddhism, Islam, Christianity, Daoism, Hinduism GDP (current US$) (billion) 298 GNI per capita, PPP (current international $) 76,850 GDP growth (annual %) 3.9 Agriculture, value added (% of GDP) +0 Industry, value added (% of GDP) 25 Services, etc., value added (% of GDP) 75 Brief Description Singapore is a city-state consisting of Singapore Island, which is located close to the southern edge of the Malay Peninsula, and 62 other smaller outlying islands. Singapore is ranked as the second most densely populated country in the world, after Monaco. With four languages being used as official languages, the country itself is a competitive business district. Therefore, there are many residents other than Singaporean living in the country. Singapore is one of the founding members of ASEAN (founded on August 8, 1967), and the leading economy in ASEAN. Cooperation with ASEAN countries is a basic diplomatic policy of Singapore. -

Chapter Two Marine Organisms
THE SINGAPORE BLUE PLAN 2018 EDITORS ZEEHAN JAAFAR DANWEI HUANG JANI THUAIBAH ISA TANZIL YAN XIANG OW NICHOLAS YAP PUBLISHED BY THE SINGAPORE INSTITUTE OF BIOLOGY OCTOBER 2018 THE SINGAPORE BLUE PLAN 2018 PUBLISHER THE SINGAPORE INSTITUTE OF BIOLOGY C/O NSSE NATIONAL INSTITUTE OF EDUCATION 1 NANYANG WALK SINGAPORE 637616 CONTACT: [email protected] ISBN: 978-981-11-9018-6 COPYRIGHT © TEXT THE SINGAPORE INSTITUTE OF BIOLOGY COPYRIGHT © PHOTOGRAPHS AND FIGURES BY ORINGAL CONTRIBUTORS AS CREDITED DATE OF PUBLICATION: OCTOBER 2018 EDITED BY: Z. JAAFAR, D. HUANG, J.T.I. TANZIL, Y.X. OW, AND N. YAP COVER DESIGN BY: ABIGAYLE NG THE SINGAPORE BLUE PLAN 2018 ACKNOWLEDGEMENTS The editorial team owes a deep gratitude to all contributors of The Singapore Blue Plan 2018 who have tirelessly volunteered their expertise and effort into this document. We are fortunate to receive the guidance and mentorship of Professor Leo Tan, Professor Chou Loke Ming, Professor Peter Ng, and Mr Francis Lim throughout the planning and preparation stages of The Blue Plan 2018. We are indebted to Dr. Serena Teo, Ms Ria Tan and Dr Neo Mei Lin who have made edits that improved the earlier drafts of this document. We are grateful to contributors of photographs: Heng Pei Yan, the Comprehensive Marine Biodiversity Survey photography team, Ria Tan, Sudhanshi Jain, Randolph Quek, Theresa Su, Oh Ren Min, Neo Mei Lin, Abraham Matthew, Rene Ong, van Heurn FC, Lim Swee Cheng, Tran Anh Duc, and Zarina Zainul. We thank The Singapore Institute of Biology for publishing and printing the The Singapore Blue Plan 2018. -

60 Years of National Development in Singapore
1 GROUND BREAKING 60 Years of National Development in Singapore PROJECT LEADS RESEARCH & EDITING DESIGN Acknowledgements Joanna Tan Alvin Pang Sylvia Sin David Ee Stewart Tan PRINTING This book incorporates contributions Amit Prakash ADVISERS Dominie Press Alvin Chua from MND Family agencies, including: Khoo Teng Chye Pearlwin Koh Lee Kwong Weng Ling Shuyi Michael Koh Nicholas Oh Board of Architects Ong Jie Hui Raynold Toh Building and Construction Authority Michelle Zhu Council for Estate Agencies Housing & Development Board National Parks Board For enquiries, please contact: Professional Engineers Board The Centre for Liveable Cities Urban Redevelopment Authority T +65 6645 9560 E [email protected] Printed on Innotech, an FSC® paper made from 100% virgin pulp. First published in 2019 © 2019 Ministry of National Development Singapore All rights reserved. No part of this publication may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of the copyright owners. Every effort has been made to trace all sources and copyright holders of news articles, figures and information in this book before publication. If any have been inadvertently overlooked, MND will ensure that full credit is given at the earliest opportunity. ISBN 978-981-14-3208-8 (print) ISBN 978-981-14-3209-5 (e-version) Cover image View from the rooftop of the Ministry of National Development building, illustrating various stages in Singapore’s urban development: conserved traditional shophouses (foreground), HDB blocks at Tanjong Pagar Plaza (centre), modern-day public housing development Pinnacle@Duxton (centre back), and commercial buildings (left). -

Caring for Our People: 50 Years of Healthcare in Singapore
Caring for our People Prime Minister’s Message Good health is important for individuals, for families, and for our society. It is the foundation for our people’s vitality and optimism, and a reflection of our nation’s prosperity and success. A healthy community is also a happy one. Singapore has developed our own system for providing quality healthcare to all. Learning from other countries and taking advantage of a young population, we invested in preventive health, new healthcare facilities and developing our healthcare workforce. We designed a unique financing system, where individuals receive state subsidies for public healthcare but at the same time can draw upon the 3Ms – Medisave, MediShield and Medifund – to pay for their healthcare needs. As responsible members of society, each of us has to save for our own healthcare needs, pay our share of the cost, and make good and sensible decisions about using healthcare services. Our healthcare outcomes are among the best in the world. Average life expectancy is now 83 years, compared with 65 years in 1965. The infant mortality rate is 2 per 1,000 live births, down from 26 per 1,000 live births 50 years ago. This book is dedicated to all those in the Government policies have adapted to the times. We started by focusing on sanitation and public health and went on healthcare sector who laid the foundations to develop primary, secondary and tertiary health services. In recent years, we have enhanced government subsidies of a healthy nation in the years gone by, substantially to ensure that healthcare remains affordable. -

CONTRACTS EXECUTED by YENG TONG CONSTRUCTION PTE LTD * All Amounts Are in SGD Unless Otherwise Stated
CONTRACTS EXECUTED BY YENG TONG CONSTRUCTION PTE LTD * All Amounts are in SGD unless otherwise stated. YENG TONG'S SCOPE OF TITLE YEAR CLIENT MAIN CONTRACTOR WORKS Proposed Construction and Completion of East Coast Economic July 2012 ~ Jan Pembinaan Yeng Tong Sdn Coastal Protection Works at Teluk Lipat, Shore Protection Works Region Development 2015 Bhd Dungun, Terengganu Darul Iman Council (ECERDC) Proposed Construction and Completion of East Coast Economic Aug 2012 ~ Nov Pembinaan Yeng Tong Sdn Coastal Protection Works at Tanjung Shore Protection Works Region Development 2012 Bhd Batu, Pekan, Pahang Darul Makmur Council (ECERDC) Maintenance dredging at tanjong pagar, 26/11/2012~ PSA CORPORATION Yeng Tong Construction PJ105 Dredging Works keppel and brani terminal defined areas 25/08/2013 LIMITED Pte Ltd Shore Protection Work for Reclaimation Hyundai Engineering & PJ104 Reclamation works Aug 2012~ JTC Corporation Work At RRM V/O Construction Co. Ltd Penta Ocean Construction Koon Construction & PJ103 Reclamation of T-Bund at Jurong Island Reclamation works May 2012 ~ Pte Ltd Transport Co. Pte Ltd. Reclaimation of Jurong Shore Protection Work for Reclaimation Hyundai Engineering & PJ102 Island Phrase 4 % Tuas View Apr 2012 ~ JTC Corporation Work At A2 - A3a Corner V/O Construction Co. Ltd Extension Option 1-1 Feb 2012~ Yeng Tong Construction PJ101 Proposed Dredging of Benoi Basin Dredging Works JTC Corporation Jun 2012 Pte Ltd Reclamation of Jurong Island PH4 & Tuas Sep 2011~ Hyundai Engineering & PJ100 View Extension Shore Protection Works Shore Protection Works JTC Corporation Mar 2012 Construction Co. Ltd For New Yard Phase 2 V.O. Reclamation of Jurong Island Phase 4 & Shore Protection Works at Sept 2011~ Hyundai Engineering & PJ099 JTC Corporation Tuas View Extension - Option 1-1 New Yard Phase 2 Feb 2012 Construction Co. -

Building an Offshore Nature Haven on Trash
CASE STUDY Building an Offshore Nature Haven on Trash Landfills are associated with toxic environments, yet the offshore landfill in Singapore has thriving marine life that people come to explore during low tides. Photo credit: Ria Tan. Singapore built an offshore landfill on Pulau Semakau primarily for waste management, but it also ensured that marine life would thrive and it could serve as a public park. Published: 27 November 2017 Overview As a fast-growing island city-state, Singapore has little space on its mainland for the rubbish its residents generate. Its innovative solution was to build one of the world’s earliest and cleanest offshore landfills—an island that today harbours flourishing ecosystems from mangroves to coral reefs. This case study was adapted from Urban Solutions of the Centre for Liveable Cities in Singapore. Challenges In 1992, one of Singapore’s last two landfill sites, a dumping ground at Lim Chu Kang in the north western region of the mainland, reached its maximum capacity and was closed. The other landfill, a 234- hectare site at Lorong Halus in the northeast, was expected to fill up by 1999. Singapore, like any small country or city with a burgeoning economy, faced a trash problem. As it grew, it generated increasing amounts of waste. In 1970, about 1,300 tonnes of solid waste was disposed of each day; by 1992, this had ballooned to about 6,000 tonnes a day. This was despite the fact that Singapore had been incinerating its trash since the late 1970s to generate energy and minimise the land required for dumps. -

One Party Dominance Survival: the Case of Singapore and Taiwan
One Party Dominance Survival: The Case of Singapore and Taiwan DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Lan Hu Graduate Program in Political Science The Ohio State University 2011 Dissertation Committee: Professor R. William Liddle Professor Jeremy Wallace Professor Marcus Kurtz Copyrighted by Lan Hu 2011 Abstract Can a one-party-dominant authoritarian regime survive in a modernized society? Why is it that some survive while others fail? Singapore and Taiwan provide comparable cases to partially explain this puzzle. Both countries share many similar cultural and developmental backgrounds. One-party dominance in Taiwan failed in the 1980s when Taiwan became modern. But in Singapore, the one-party regime survived the opposition’s challenges in the 1960s and has remained stable since then. There are few comparative studies of these two countries. Through empirical studies of the two cases, I conclude that regime structure, i.e., clientelistic versus professional structure, affects the chances of authoritarian survival after the society becomes modern. This conclusion is derived from a two-country comparative study. Further research is necessary to test if the same conclusion can be applied to other cases. This research contributes to the understanding of one-party-dominant regimes in modernizing societies. ii Dedication Dedicated to the Lord, Jesus Christ. “Counsel and sound judgment are mine; I have insight, I have power. By Me kings reign and rulers issue decrees that are just; by Me princes govern, and nobles—all who rule on earth.” Proverbs 8:14-16 iii Acknowledgments I thank my committee members Professor R. -

NSS Bird Group Report – Jun 2016
NSS Bird Group Report – Jun 2016 The forest Blue-eared Kingfisher fishing at the canal at Kranji Marshes. Photo: Mark Nelson Valino. There were less excitement this month compared to last June. Then we had the first record of a White-tailed Tropicbird at Tuas, an Oriental Darter at Ubin and the wintering Horsfield’s Bronze Cuckoos at Punggol Barat to keep us busy. This June, we had to be contented with the King Quails, Excalfactoria chinensis, at Punggol Barat, Blue- eared Kingfishers, Alcedo meninting, at Kranji Marshes and Red- crowned Barbets, Megalaima rafflesii at Seletar to keep our shutters clicking. On the plus side we had many interesting nesting records including a first from all over the island. Wendy Lin and friends Peter Okimi, Chai Lee Fung and Edwin Choy were at Seletar Revervoir Park on 30th May looking for the Chestnut-winged Babblers when they chanced upon the Red-crowned Barbets feeding on the fruits of the Green Coffee Tree. Photo: Wendy Lin showing the barbet squeezing the pulp out of the fruit. 1 The most surprising record came from Cashew area on 20th when a member of the public reported a baby owl at the foot of a rain tree on her way to work. NParks staff rescued the owlet and subsequently put it back into its Bird-nest Fern nest much to the relief of a pair of Spotted Wood Owl, Strix seloputo, parents. This is a rare nesting record for this uncommon owl and confirmed that they also use Bird- nest Ferns to nest just like the Buffy Fish Owls. -

NATIONAL PRIDE Diverse Influences That Have Broadened Horizons, Cities That Are a Melting Pot of Cultures + Culinary Traditions That Make Singapore Unique
JULY/AUGUST 2021 A PUBLICATION OF ONE°15 MARINA SENTOSA COVE SINGAPORE NATIONAL PRIDE Diverse influences that have broadened horizons, cities that are a melting pot of cultures + Culinary traditions that make Singapore unique ALL ABOARD TIES THAT BIND e start the third quarter of 2021 on We are happy to be celebrating these achievements alongside Singapore’s a high. ONE°15 Marina Sentosa 56th National Day. This year reminds us to be especially grateful for a Cove has won the International nation that is able to keep its people safe. As a family-oriented Club, it WMarina of the Year 2021 award by has been our priority to keep our Members and staff safe, and following Marina Industries Association (MIA). This is in all COVID-19 safety protocols has been a part of that process. We are recognition of our international-standard marina grateful for our Members’ understanding through this difficult journey. facilities, exemplary business practices, commitment Your support has enabled us to keep up the vibe of special events—the to service and environmental focus—all things that recent Mother’s and Father’s Day celebrations at the are part of the Club’s DNA. WE AIM TO Club were among those. Our commitment to sustainability and to protect CAPTURE When we talk about Singapore, it is more than our inland and coastal waterways is a big part of just a city-state that proffers a luxurious lifestyle, that. The recent re-accreditation as a Level 4 Clean THAT TRUE it’s a country that has been threaded together by Marina by MIA affirms that pledge, keeping us even ESSENCE OF the traditions, practices and cultures of different more focused on our end goals. -
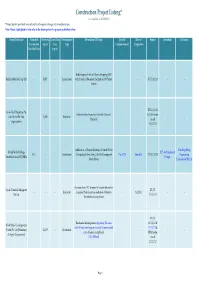
Construction Project Listing* (Last Updated on 20/12/2013) *Project Details Provided May Subject to Subsequent Changes by Owner/Developer
Construction Project Listing* (Last Updated on 20/12/2013) *Project details provided may subject to subsequent changes by owner/developer. Note: Words highlighted in blue refer to the latest updates for projects published before. Owner/Developer Estimated Site Area Gross Floor Development Description Of Project Date Of Date of Source Consultant Contractor Construction (sq m) Area Type Commencement Completion Cost ($million) (sq m) Redevelopment into a 6 Storey Shopping Mall Raffles Medical Group Ltd - 5,827 - Institutional with 2 levels of Basement Carpark at 100 Taman - - ST 17/12/13 - - Warna BT 11/12/13 Grow-Tech Properties Pte Industrial development at Gambas Crescent & URA tender Ltd (Part of Far East - 14,302 - Industrial -- -- (Parcel 3) result Organization) 13/12/13 Addition of a 5 Storey Building to United World Kim Seng Heng United World College BLT Architecture & 42.5 - - Institutional College South East Asia (UWCSEA) campus at Dec-2013 Aug-2015 BT 13/12/13 Engineering South East Asia (UWCSEA) Design Dover Road Construction Pte Ltd Erection of an LPG Terminal to import alternative Vopak Terminals Singapore BT/ST - - - Industrial Liquefied Petroleum Gas feedstock at Banyan - 1Q 2016 -- Pte Ltd 11/12/13 Terminal in Jurong Island BT/ST Residential development comprising 281 units 16/11/12 & World Class Developments with 24 hour concierge service and 18 commercial 11/12/13 & (North) Pte Ltd [Subsidiary - 10,170 - Residential -- -- units at Jalan Jurong Kechil URA tender of Aspial Corporation] (The Hillford) result 22/11/12 Page 1 Construction Project Listing* (Last Updated on 20/12/2013) *Project details provided may subject to subsequent changes by owner/developer. -

Singapore's Reclamation Story
BIBLIOASIA APR – JUN 2017 Vol. 13 / Issue 01 / Feature Lim Tin Seng is a Librarian with the National (Facing page) Aerial photograph of ongoing reclamation work in Tuas. Photo by Richard W. J. Koh. All rights Library, Singapore. He is the co-editor of reserved, Koh, T. (2015). Over Singapore (pp. 108–109). Singapore: Editions Didier Millet. Roots: Tracing Family Histories – A Resource (Below) This lithograph (c. 1850) by Lieutenant Edwin Augustus Porcher from the British Royal Navy Guide (2013), Harmony and Development: shows the view as seen from South Boat Quay, where Singapore’s first reclamation took place in 1822. ASEAN-China Relations (2009) and Courtesy of the National Museum of Singapore, National Heritage Board. (Bottom) Named after George Chancellor Collyer, then Chief Engineer of the Straits Settlements, Collyer China’s New Social Policy: Initiatives for Quay was built on reclaimed land by convict labour and completed in 1864. Courtesy of National Archives a Harmonious Society (2010). He is also a of Singapore. LAND regular contributor to BiblioAsia. FROM Over the past two centuries, Singapore’s land area has expanded by a whopping 25 percent – from 58,150 to 71,910 hectares (or 578 to 719 sq km).1 This gradual increase in land surface is not because of tectonic movements or divine intervention, but SAND orather the miracle of a man-made engi- Singapore’s Reclamation Story neering feat known as land reclamation. The quest for land is as old as time immemorial; one of the reasons nations go to war is to gain new territory to sup- port a growing population.