Are Dried and Powdered Moringa Oleifera Lam. Leaves Susceptible to Moths That Feed on Stored Products?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
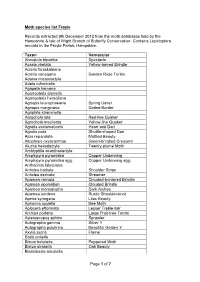
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Descripción De Los Huevos Y De Las Estrategias De Puesta De Seis Taxones De Sterrhinae De Madrid, Con Datos Comparativos De
Graellsia, 72(1): e041 enero-junio 2016 ISSN-L: 0367-5041 http://dx.doi.org/10.3989/graellsia.2016.v72.146 DESCRIPTION OF THE OVA AND OVIPOSITIONAL STRATEGIES OF SIX STERRHINE TAXA FROM MADRID, INCLUDING COMPARATIVE DATA WITH OTHER SPECIES OF THIS SUBFAMILY (LEPIDOPTERA: GEOMETRIDAE: STERRHINAE) Gareth Edward King* & José Luis Viejo Montesinos Departamento de Biología (Zoología), Universidad Autónoma de Madrid, C/. Darwin, 2, 28049 Cantoblanco (Madrid) * Corresponding author: [email protected] ABSTRACT Original data are presented which describe ova of the following six taxa in the Sterrhinae Meyrick, 1892: Idaea litigiosaria (Boisduval, 1840), Idaea sericeata calvaria Wehrli, 1927, Idaea ochrata albida (Zerny, 1936), Idaea incisaria (Staudinger, 1892), Idaea cervantaria (Millière, 1869) and Scopula (Glossotrophia) asellaria dentatolin- eata Wehrli, 1926. Subsequent analysis of SEM imaging provides data related to the chorion structure, as well as that associated with the strategies adopted by females at oviposition under laboratory conditions; comparative data are provided of other European sterrhines. Key words: Lepidoptera; Geometridae; Sterrhinae; ova; morphology; biology; egg positioning. RESUMEN Descripción de los huevos y de las estrategias de puesta de seis taxones de Sterrhinae de Madrid, con datos comparativos de otras especies de la subfamilia (Lepidoptera: Geometridae: Sterrhinae) Se presentan datos inéditos que describen los huevos de los siguientes taxones de Sterrhinae Meyrick, 1892: Idaea litigiosaria (Boisduval, 1840), Idaea sericeata calvaria Wehrli, 1927, Idaea ochrata albida (Zerny, 1936), Idaea incisaria (Staudinger, 1892), Idaea cervantaria (Millière, 1869) and Scopula (Glossotrophia) ase- llaria dentatolineata Wehrli, 1926. Se ofrece un análisis pormenorizado de las imágenes MEB en cuanto a la estructura del corión se refiere, además de una descripción de las estrategias de la puesta adoptadas por parte de las hembras en condiciones de laboratorio. -

Study of the Postembryonic Development of Idaea Inquinata Under Different Abiotic Factors
Bulletin of Insectology 66 (1): 21-25, 2013 ISSN 1721-8861 Study of the postembryonic development of Idaea inquinata under different abiotic factors Lidia LIMONTA, Daria P. LOCATELLI Dipartimento di Scienze per gli alimenti, la nutrizione e l'ambiente (DeFENS), Università degli Studi di Milano, Italy Abstract We investigated the biology of Idaea inquinata (Scopoli) (Lepidoptera Geometridae), the rusty wave moth, to determine the num- ber and duration of larval instars and the duration of the pupal stage. The study was conducted at 21, 26, 29, and 34 ± 1 °C; for each temperature tests were conducted at 35 and 70 ± 5% relative humidity (RH), with a photoperiod of 16:8 (light:dark). At 35% RH, five larval instars were observed at 26, 29, and 34 °C, whereas eight instars were found at 21 °C. At 21 °C and 70% RH, only one larva pupated after the fifth instar, two completed the sixth instar and one reached the seventh instar. At 21 °C and 35% RH an increase of mortality and in the number of larval instars was observed; the few larvae that reached the tenth instar did not survive. The shortest larval developmental periods were observed at 26 and 29 °C at 70% RH and were 31.9 ± 2.26 and 30.6 ± 3.12 days respectively. The longest developmental period was recorded at 21 °C and 35% RH, and was 172.5 ± 16.26 days. The pupal stage was longer at 21 °C at 35 and 70% RH, and lasted 22.5 ± 2.12 and 22 ± 3.65 days respectively. -

CHECKLIST of WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea)
WISCONSIN ENTOMOLOGICAL SOCIETY SPECIAL PUBLICATION No. 6 JUNE 2018 CHECKLIST OF WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea) Leslie A. Ferge,1 George J. Balogh2 and Kyle E. Johnson3 ABSTRACT A total of 1284 species representing the thirteen families comprising the present checklist have been documented in Wisconsin, including 293 species of Geometridae, 252 species of Erebidae and 584 species of Noctuidae. Distributions are summarized using the six major natural divisions of Wisconsin; adult flight periods and statuses within the state are also reported. Examples of Wisconsin’s diverse native habitat types in each of the natural divisions have been systematically inventoried, and species associated with specialized habitats such as peatland, prairie, barrens and dunes are listed. INTRODUCTION This list is an updated version of the Wisconsin moth checklist by Ferge & Balogh (2000). A considerable amount of new information from has been accumulated in the 18 years since that initial publication. Over sixty species have been added, bringing the total to 1284 in the thirteen families comprising this checklist. These families are estimated to comprise approximately one-half of the state’s total moth fauna. Historical records of Wisconsin moths are relatively meager. Checklists including Wisconsin moths were compiled by Hoy (1883), Rauterberg (1900), Fernekes (1906) and Muttkowski (1907). Hoy's list was restricted to Racine County, the others to Milwaukee County. Records from these publications are of historical interest, but unfortunately few verifiable voucher specimens exist. Unverifiable identifications and minimal label data associated with older museum specimens limit the usefulness of this information. Covell (1970) compiled records of 222 Geometridae species, based on his examination of specimens representing at least 30 counties. -

Impact of Temperature and Relative Humidity on the Eye-Spotted Bud Moth, Spilonota Ocellana (Lepidoptera: Tortricidae): a Climate Change Perspective
Impact of temperature and relative humidity on the eye-spotted bud moth, Spilonota ocellana (Lepidoptera: Tortricidae): a climate change perspective by Jolene Ann Swain B.Sc., University of Alberta, 2008 Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Department of Biological Sciences Faculty of Science © Jolene A. Swain 2016 SIMON FRASER UNIVERSITY Spring 2016 Approval Name: Jolene Ann Swain Degree: Master of Science Title: Impact of temperature and relative humidity on the eye-spotted bud moth, Spilonota ocellana (Lepidoptera: Tortricidae): a climate change perspective Examining Committee: Chair: Dr. Vicky Marlatt Assistant Professor Dr. Jenny Cory Senior Supervisor Professor Dr. Tony Williams Supervisor Professor Dr. Dave Gillespie Supervisor Research Scientist, Agriculture and Agri-Food Canada Dr. Gary Judd Supervisor Research Scientist, Agriculture and Agri-Food Canada Dr. Gerhard Gries External Examiner Professor Date Defended/Approved: April 15, 2016 ii Abstract Global climate change models predict an increase in the frequency, severity and duration of extreme weather events. Weather extremes are important for poikilothermic species limited by their capacity to withstand conditions beyond their optimum for survival and development. To understand insect population dynamics, and forecast outbreaks in agro-ecosystems, we need a better understanding of the biology of insect pests of concern. In this study, I explored physiological responses of Spilonota ocellana (Denis and Schiffermüller) in the context of spring frost and summer drought, by focusing on the most vulnerable life stages. I determined that S. ocellana spring larval instars are susceptible to temperatures above their mean supercooling point (SCP) which ranged from -9.1 ± 0.2 °C (4th instar) to -7.9 ± 0.2 °C (6th instar). -

<I>Tribolium Castaneum</I>
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln U.S. Department of Agriculture: Agricultural Publications from USDA-ARS / UNL Faculty Research Service, Lincoln, Nebraska 2015 Susceptibility of Tribolium castaneum (Coleoptera: Tenebrionidae) and Trogoderma inclusum (Coleoptera: Dermestidae) to cold temperatures Frank H. Arthur U.S. Grain Marketing and Production Research Center, [email protected] K. L. Hartzer USDA-ARS James E. Throne USDA-ARS, Manhattan, KS, [email protected] P. W. Flinn Retired Follow this and additional works at: https://digitalcommons.unl.edu/usdaarsfacpub Arthur, Frank H.; Hartzer, K. L.; Throne, James E.; and Flinn, P. W., "Susceptibility of Tribolium castaneum (Coleoptera: Tenebrionidae) and Trogoderma inclusum (Coleoptera: Dermestidae) to cold temperatures" (2015). Publications from USDA-ARS / UNL Faculty. 2055. https://digitalcommons.unl.edu/usdaarsfacpub/2055 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Agricultural Research Service, Lincoln, Nebraska at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications from USDA-ARS / UNL Faculty by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Journal of Stored Products Research 64 (2015) 45e53 Contents lists available at ScienceDirect Journal of Stored Products Research -

Pheromone Lures Non-Intended Target Species
Updated July 2021 ALS ALS have been selling pheromone lures since 2004 and started with the Clearwing Classic Six. Over the years we have extended our range with new species, some of which have been made specifically to our demand while also adding lures for various other species of moths. We have collated all our data, much of which have been passed on to us by many customers of which we thank very much especially Tim Green and Graham Ekins (Essex), to produce the below non-target species data base. Pheromone lures are made up of between 4-6 compounds and it is some of these ‘base’ compounds which are acting as an attractant to other species. Although some of the species listed are quite rare to synthetic lure, others do seem to be more frequent. The below data is from the UK and Europe. If you have any additional information, please get in touch with us. SPECIES and lure code Non-intended moth target species caught Six-belted Clearwing - Bembecia ichneumoniformis API Bembecia albanensis Five-spot Burnet sp. Six-spot Burnet Nemapogon variatella Pyropteron triannuliformis Chamaesphecia empiformis Pyropteron chrysidiformis Thrift/Raspberry Clearwing HYL Bembecia ichneumoniformis (Six-belted Clearwing) Synanthedon conopiformis (Oak Clearwing) Phalonidia affinitana Stenoptinea cyaneimarmorella Synansphecia muscaeformis/Pennisetia hylaeiformis Cacaecimorpha pronubana Lobesia littoralis Cnephasia pumicana Dryadaula heindeli Hypsopygia costalis (Gold Triangle) Lymantria dispar (Gypsy Moth) Nemapogon ruricolella Pseudargyrotoza conwagana Opisthograptis -

Ajay Kumar Tiwari Editor Advances in Seed Production and Management Advances in Seed Production and Management Ajay Kumar Tiwari Editor
Ajay Kumar Tiwari Editor Advances in Seed Production and Management Advances in Seed Production and Management Ajay Kumar Tiwari Editor Advances in Seed Production and Management Editor Ajay Kumar Tiwari UP Council of Sugarcane Research Shahjahanpur, Uttar Pradesh, India ISBN 978-981-15-4197-1 ISBN 978-981-15-4198-8 (eBook) https://doi.org/10.1007/978-981-15-4198-8 # Springer Nature Singapore Pte Ltd. 2020 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. This Springer imprint is published by the registered company Springer Nature Singapore Pte Ltd. -

Beginner S Guide to Moths of the Midwest Geometers
0LGZHVW5HJLRQ86$ %HJLQQHU V*XLGHWR0RWKVRIWKH0LGZHVW*HRPHWHUV $QJHOOD0RRUHKRXVH ,OOLQRLV1DWXUH3UHVHUYH&RPPLVVLRQ Photos: Angella Moorehouse ([email protected]). Produced by: Angella Moorehouse with the assistance of Alicia Diaz, Field Museum. Identification assistance provided by: multiple sources (inaturalist.org; bugguide.net) )LHOG0XVHXP &&%<1&/LFHQVHGZRUNVDUHIUHHWRXVHVKDUHUHPL[ZLWKDWWULEXWLRQEXWFRPPHUFLDOXVHRIWKHRULJLQDOZRUN LVQRWSHUPLWWHG >ILHOGJXLGHVILHOGPXVHXPRUJ@>@YHUVLRQ $ERXWWKH%(*,11(5¶6027+62)7+(0,':(67*8,'(6 Most photos were taken in west-central and central Illinois; a fewDUH from eastern Iowa and north-central Wisconsin. Nearly all were posted to identification websites: BugGuide.netDQG iNaturalist.org. Identification help was provided by Aaron Hunt, Steve Nanz, John and Jane Balaban, Chris Grinter, Frank Hitchell, Jason Dombroskie, William H. Taft, Jim Wiker,DQGTerry Harrison as well as others contributing to the websites. Attempts were made to obtain expert verifications for all photos to the field identification level, however, there will be errors. Please contact the author with all corrections Additional assistance was provided by longtime Lepidoptera survey partner, Susan Hargrove. The intention of these guides is to provide the means to compare photographs of living specimens of related moths from the Midwest to aid the citizen scientists with identification in the field for Bio Blitz, Moth-ers Day, and other night lighting events. A taxonomic list to all the species featured is provided at the end along with some field identification tips. :(%6,7(63529,',1*,'(17,),&$7,21,1)250$7,21 BugGuide.net LNaturalist.org Mothphotographersgroup.msstate.edu Insectsofiowa.org centralillinoisinsects.org/weblog/resources/ :+,&+027+*8,'(7286( The moths were split into 6 groups for the purposes of creating smaller guides focusing on similar features of 1 or more superfamilies. -

Coastal Sage Scrub at University of California, Los Angeles
BIOLOGICAL ASSESSMENT: COASTAL SAGE SCRUB AT UNIVERSITY OF CALIFORNIA, LOS ANGELES Prepared by: Geography 123: Bioresource Management UCLA Department of Geography, Winter 1996 Dr. Rudi Mattoni Robert Hill Alberto Angulo Karl Hillway Josh Burnam Amanda Post John Chalekian Kris Pun Jean Chen Julien Scholnick Nathan Cortez David Sway Eric Duvernay Alyssa Varvel Christine Farris Greg Wilson Danny Fry Crystal Yancey Edited by: Travis Longcore with Dr. Rudi Mattoni, Invertebrates Jesus Maldonado, Mammals Dr. Fritz Hertel, Birds Jan Scow, Plants December 1, 1997 TABLE OF CONTENTS CHAPTER 1: INTRODUCTION ..........................................................................................................................1 CHAPTER 2: PHYSICAL DESCRIPTION ........................................................................................................2 GEOLOGICAL FRAMEWORK.....................................................................................................................................2 LANDFORMS AND SOILS ..........................................................................................................................................2 The West Terrace ...............................................................................................................................................3 Soil Tests.............................................................................................................................................................4 SLOPE, EROSION, AND RUNOFF ..............................................................................................................................4 -

Description of the Ova and Ovipositional Strategies Of
Graellsia, 72(1): e041 enero-junio 2016 ISSN-L: 0367-5041 http://dx.doi.org/10.3989/graellsia.2016.v72.146 DESCRIPTION OF THE OVA AND OVIPOSITIONAL STRATEGIES OF SIX STERRHINE TAXA FROM MADRID, INCLUDING COMPARATIVE DATA WITH OTHER SPECIES OF THIS SUBFAMILY (LEPIDOPTERA: GEOMETRIDAE: STERRHINAE) Gareth Edward King* & José Luis Viejo Montesinos Departamento de Biología (Zoología), Universidad Autónoma de Madrid, C/. Darwin, 2, 28049 Cantoblanco (Madrid) * Corresponding author: [email protected] ABSTRACT Original data are presented which describe ova of the following six taxa in the Sterrhinae Meyrick, 1892: Idaea litigiosaria (Boisduval, 1840), Idaea sericeata calvaria Wehrli, 1927, Idaea ochrata albida (Zerny, 1936), Idaea incisaria (Staudinger, 1892), Idaea cervantaria (Millière, 1869) and Scopula (Glossotrophia) asellaria dentatolin- eata Wehrli, 1926. Subsequent analysis of SEM imaging provides data related to the chorion structure, as well as that associated with the strategies adopted by females at oviposition under laboratory conditions; comparative data are provided of other European sterrhines. Key words: Lepidoptera; Geometridae; Sterrhinae; ova; morphology; biology; egg positioning. RESUMEN Descripción de los huevos y de las estrategias de puesta de seis taxones de Sterrhinae de Madrid, con datos comparativos de otras especies de la subfamilia (Lepidoptera: Geometridae: Sterrhinae) Se presentan datos inéditos que describen los huevos de los siguientes taxones de Sterrhinae Meyrick, 1892: Idaea litigiosaria (Boisduval, 1840), Idaea sericeata calvaria Wehrli, 1927, Idaea ochrata albida (Zerny, 1936), Idaea incisaria (Staudinger, 1892), Idaea cervantaria (Millière, 1869) and Scopula (Glossotrophia) ase- llaria dentatolineata Wehrli, 1926. Se ofrece un análisis pormenorizado de las imágenes MEB en cuanto a la estructura del corión se refiere, además de una descripción de las estrategias de la puesta adoptadas por parte de las hembras en condiciones de laboratorio. -

21 Locatelli__
REDIA, 102, 2019: 149-152 http://dx.doi.org/10.19263/REDIA-102.19.21 DARIA PATRIZIA LOCATELLI a - FATIMA BEATRIZ PEREZ GARCIA a - LIDIA LIMONTA a THE EFFECTS OF COMPETITION BETWEEN LARVAE OF STORED-PRODUCT MOTHS a Department of Food, Environmental and Nutritional Sciences, Università degli Studi di Milano, Via Giovanni Celoria 2, 20133 Milano, Italy. Corresponding Author: Lidia Limonta; [email protected] Locatelli D.P., Perez Garcia F.B., Limonta L. – The effects of competition between larvae of stored-product moths. The competition between Idaea inquinata, Corcyra cephalonica, and Plodia interpunctella on an artificial diet was investigated. The experiments were carried out with eggs laid within 24 hours. In the first experiment, 20 eggs of one species were placed in a ventilated Petri dish with 10 g of diet and 20 eggs of one of the other species; in the second experiment, 20 eggs of one species and, after 7 days, 20 eggs of one of the other species were added. Experiments were carried out at 27±1 °C, 70±5% R.H. Each experiment was replicated 5 times and the number of days to complete the development and the number of emerged adults were recorded. The number of P. interpunctella adults was not affected by the presence of C. cephalonica or I. inquinata. A delay in the development was only observed when P. interpunctella eggs were added to the medium already colonized by the other species. I. inquinata was the less competitive, as the number of adults decreased and the days to complete the cycle increased.