E504.Full.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
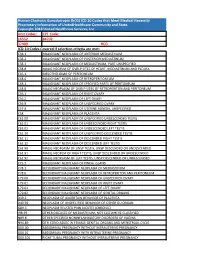
Human Chorionic Gonadotropin (Hcg) ICD 10 Codes That Meet Medical Necessity Proprietary Information of Unitedhealthcare Community and State
Human Chorionic Gonadotropin (hCG) ICD 10 Codes that Meet Medical Necessity Proprietary Information of UnitedHealthcare Community and State. Copyright 2018 United Healthcare Services, Inc Unit Codes: CPT Code: 16552 84702 37409 HCG ICD-10 Codes Covered if selection criteria are met: C38.1 MALIGNANT NEOPLASM OF ANTERIOR MEDIASTINUM C38.2 MALIGNANT NEOPLASM OF POSTERIOR MEDIASTINUM C38.3 MALIGNANT NEOPLASM OF MEDIASTINUM, PART UNSPECIFIED C38.8 MALIG NEOPLM OF OVRLP SITES OF HEART, MEDIASTINUM AND PLEURA C45.1 MESOTHELIOMA OF PERITONEUM C48.0 MALIGNANT NEOPLASM OF RETROPERITONEUM C48.1 MALIGNANT NEOPLASM OF SPECIFIED PARTS OF PERITONEUM C48.8 MALIG NEOPLASM OF OVRLP SITES OF RETROPERITON AND PERITONEUM C56.1 MALIGNANT NEOPLASM OF RIGHT OVARY C56.2 MALIGNANT NEOPLASM OF LEFT OVARY C56.9 MALIGNANT NEOPLASM OF UNSPECIFIED OVARY C57.4 MALIGNANT NEOPLASM OF UTERINE ADNEXA, UNSPECIFIED C58 MALIGNANT NEOPLASM OF PLACENTA C62.00 MALIGNANT NEOPLASM OF UNSPECIFIED UNDESCENDED TESTIS C62.01 MALIGNANT NEOPLASM OF UNDESCENDED RIGHT TESTIS C62.02 MALIGNANT NEOPLASM OF UNDESCENDED LEFT TESTIS C62.10 MALIGNANT NEOPLASM OF UNSPECIFIED DESCENDED TESTIS C62.11 MALIGNANT NEOPLASM OF DESCENDED RIGHT TESTIS C62.12 MALIGNANT NEOPLASM OF DESCENDED LEFT TESTIS C62.90 MALIG NEOPLASM OF UNSP TESTIS, UNSP DESCENDED OR UNDESCENDED C62.91 MALIG NEOPLM OF RIGHT TESTIS, UNSP DESCENDED OR UNDESCENDED C62.92 MALIG NEOPLASM OF LEFT TESTIS, UNSP DESCENDED OR UNDESCENDED C75.3 MALIGNANT NEOPLASM OF PINEAL GLAND C78.1 SECONDARY MALIGNANT NEOPLASM OF MEDIASTINUM C78.6 SECONDARY -

Histomorphological Study of Chorionic Villi in Products of Conception Following First Trimester Abortions
November, 2018/ Vol 4/ Issue 7 Print ISSN: 2456-9887, Online ISSN: 2456-1487 Original Research Article Histomorphological study of chorionic villi in products of conception following first trimester abortions Shilpa MD1, Supreetha MS2, Varshashree3 1Dr. Shilpa, MD, Assistant Professor, 2Dr. Supreetha, MS, Assistant Professor, 3Dr. Varshashree, Post graduate, all authors are affiliated with Department of Pathology, Sri Devaraj Urs Medical College, Tamaka, Kolar, Karnataka, India. Corresponding Author: Dr. Shilpa, MD, Assistant Professor, Department of Pathology, Sri Devaraj Urs Medical College, Tamaka, Kolar, Karnataka, India. Email id: [email protected] ………………………………………………………………………………………………………………………………... Abstract Background: The common problem which occurs in first trimester of pregnancy is miscarriage. Retained products of conception are commonly received specimen for histopathological examination. Apart from confirmation of pregnancy, a careful examination can provide some additional information about the cause or the conditions associated with abortion. Aim: 1. To study various histopathological changes occurring in chorionic villi in first trimester spontaneous abortions and to know the pathogenesis of abortions. Materials and methods: This was a cross sectional retrospective study carried out for over a period of 3 years from January 2015 to January 2018. A total of 235biopsies were obtained from patient with the diagnosis of the first trimester spontaneous abortions were included in this study. Results: In our study most common age group of the abortion was between 21-30 years (63%). Incomplete abortion was the commonest type of abortion (47.7%). Many dysmorphic features were observed in this study like hydropic change (67%), stromal fibrosis (62%), villi with reduced blood vessels (52.7%) and perivillous fibrin deposition. -

Medical Abortion Reference Guide INDUCED ABORTION and POSTABORTION CARE at OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) © 2017, 2018 Ipas
Medical Abortion Reference Guide INDUCED ABORTION AND POSTABORTION CARE AT OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) © 2017, 2018 Ipas ISBN: 1-933095-97-0 Citation: Edelman, A. & Mark, A. (2018). Medical Abortion Reference Guide: Induced abortion and postabortion care at or after 13 weeks gestation (‘second trimester’). Chapel Hill, NC: Ipas. Ipas works globally so that women and girls have improved sexual and reproductive health and rights through enhanced access to and use of safe abortion and contraceptive care. We believe in a world where every woman and girl has the right and ability to determine her own sexuality and reproductive health. Ipas is a registered 501(c)(3) nonprofit organization. All contributions to Ipas are tax deductible to the full extent allowed by law. For more information or to donate to Ipas: Ipas P.O. Box 9990 Chapel Hill, NC 27515 USA 1-919-967-7052 [email protected] www.ipas.org Cover photo: © Ipas The photographs used in this publication are for illustrative purposes only; they do not imply any particular attitudes, behaviors, or actions on the part of any person who appears in the photographs. Printed on recycled paper. Medical Abortion Reference Guide INDUCED ABORTION AND POSTABORTION CARE AT OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) Alison Edelman Senior Clinical Consultant, Ipas Professor, OB/GYN Oregon Health & Science University Alice Mark Associate Medical Director National Abortion Federation About Ipas Ipas works globally so that women and girls have improved sexual and reproductive health and rights through enhanced access to and use of safe abortion and contraceptive care. -

Miscarriage Or Early Pregnancy Loss- Diagnosis and Management (Version 5)
Miscarriage or early pregnancy loss- diagnosis and management (Version 5) Guideline Readership This guideline applies to all women diagnosed with miscarriage in early pregnancy (up to 13 completed weeks) within the Heart of England Foundation Trust and to attending clinicians, sonographers and nursing staff on Gynaecology ward and early pregnancy unit. All care is tailored to individual patient needs, with an in-depth discussion of the intended risks and benefits for any intervention offered to woman with early pregnancy loss. Guideline Objectives The objective of this guideline is to enable all clinicians to recognise the different types of miscarriages and to follow a recognised management pathway so that all women with actual or suspected miscarriage receive, an appropriate and individualised care. Other Guidance Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guidance Dec 2012 Ratified Date: Insert Date Launch Date: 16 March 2018 Review Date: 16 March 2021 Guideline Author: Dr Rajmohan, Dr Cheema Contents & page numbers: 1. Flowcharts Flowchart 1 – Management of complete miscarriage p3 Flowchart 2 – Management of incomplete miscarriage p4 Flowchart 3 – Management of Missed miscarriage p5 Flowchart 4 - Management of Early fetal demise p6 Flowchart 5 – Medical management of miscarriage p7 Flowchart 6 - Surgical management (SMM) pathway p8 2. Executive summary and Overview p9 3. Body of guideline Types of miscarriage p9 Threatened miscarriage p9 Complete miscarriage p9 Incomplete miscarriage p9 Missed miscarriage -

Ectopic Pregnancy Management: Tubal and Interstitial SIGNS / SYMPTOMS DIAGNOSIS
8/29/19njm Ectopic Pregnancy Management: Tubal and interstitial SIGNS / SYMPTOMS Pain and vaginal bleeding are the hallmark symptoms of ectopic pregnancy. Pain is almost universal; it is generally lower abdominal and unilateral. Bleeding is also very common following a short period of amenorrhea. Physical exam may reveal a tender adnexal mass, often mentioned in texts, but noted clinically only 20 percent of the time. Furthermore, it may easily be confused with a tender corpus luteum of a normal intrauterine pregnancy. Finally signs and symptoms of hemoperitoneum and shock can occur, including a distended, silent, “doughy” abdomen, shoulder pain, bulging cul de sac into the posterior fornix of the vagina, and hypotension. DIAGNOSIS Initially, serum hCG rises, but then usually plateaus or falls. Transvaginal ultrasound scanning is a key diagnostic tool and can rapidly make these diagnoses: 1. Ectopic is ruled out by the presence of an intrauterine pregnancy with the exception of rare heterotopic pregnancy 2. Ectopic is proven when a gestational sac and an embryo with a heartbeat is seen outside of the uterus 3. Ectopic is highly likely if ANY adnexal mass distinct from the corpus luteum or a significant amount of free pelvic fluid is seen. When ultrasound is not definitive, correlation of serum hCG levels is important. If the hCG is above the “discriminatory zone” of 3000 mIU/ml IRP, a gestational sac should be visible on transvaginal ultrasound. The discriminatory zone varies by ultrasound machine and sonographer. If an intrauterine gestational sac is not visible by the time the hCG is at, or above, this threshold, the pregnancy has a high likelihood of being ectopic. -

Guide to Major Eleventh Edition Changes: Obstetrics, Neonates And
Guide to Major Eleventh Edition Changes: Obstetrics, neonates and genitourinary WA Clinical Coding Authority Purchasing and System Performance Division August 2019, revised January 2020 1 Contents Chapter 15 Pregnancy, childbirth and the puerperium 3 ACS 1500 Diagnosis sequencing in obstetric episodes of care 3 ACS 1505 Delivery and assisted delivery codes 3 Delivery of baby prior to the admitted episode of care 5 ACS 1550 Discharge/transfer in labour 7 Transfer in first stage of labour 7 Transfer in third stage of labour 7 Failed induction of labour 9 Postpartum haemorrhage 9 ACS 1551 Obstetric perineal lacerations/grazes 11 ACS 1511 Termination of pregnancy (abortion) 11 ACS 1544 Complications following pregnancy with abortive outcome 14 Complications following abortion 14 Admission for retained products of conception following abortion 16 Hypoglycaemia in gestational diabetes mellitus 16 Mental Health and pregnancy 16 Neonates 16 Genitourinary 17 Acknowledgement 17 2 This guide was updated in January 2020 with creation of Examples 6, 7 and 19. Chapter 15 Pregnancy, childbirth and the puerperium Amendments include: Changes to code titles to specify ‘in pregnancy, childbirth and the puerperium.’ Changes to ACS titles for 1500 Diagnosis sequencing in obstetric episodes of care, 1511 Termination of pregnancy (abortion) and 1544 Complications following pregnancy with abortive outcome. Deletion of Excludes notes throughout ICD-10-AM to promote multiple condition coding. Clarification that codes from other chapters may be assigned to add specificity. Creation of four character codes to classify failed medical and surgical induction of labour. Indexing amendments to clarify code assignment for failed trial of labour and failure to progress unspecified, with or without identification of an underlying cause. -
Title Management of Retained Products of Conception with Marked
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository Management of retained products of conception with marked Title vascularity. Kitahara, Tomoko; Sato, Yukiyasu; Kakui, Kazuyo; Tatsumi, Author(s) Keiji; Fujiwara, Hiroshi; Konishi, Ikuo The journal of obstetrics and gynaecology research (2011), Citation 37(5): 458-464 Issue Date 2011-01-05 URL http://hdl.handle.net/2433/197589 This is the peer reviewed version of the following article: Kitahara, T., Sato, Y., Kakui, K., Tatsumi, K., Fujiwara, H. and Konishi, I. (2011), Management of retained products of Right conception with marked vascularity. Journal of Obstetrics and Gynaecology Research, 37: 458‒464, which has been published in final form at http://dx.doi.org/10.1111/j.1447- 0756.2010.01363.x Type Journal Article Textversion author Kyoto University 1 Management of Retained Products of Conception with Marked Vascularity 2 3 Tomoko Kitahara, Yukiyasu Sato*, Kazuyo Kakui, Keiji Tatsumi, Hiroshi Fujiwara, and 4 Ikuo Konishi 5 6 Department of Gynecology and Obstetrics, Kyoto University Graduate School of 7 Medicine 8 9 *Address correspondence and reprint requests to: Yukiyasu Sato, M.D., Ph.D. 10 Department of Gynecology and Obstetrics, Kyoto University Graduate School of 11 Medicine, Sakyo-ku, Kyoto 606-8507, Japan. 12 Tel; 81-75-751-3269: Fax; 81-75-761-3967: E-mail; [email protected] 13 14 Short title; Management of Hypervascular RPOC 15 Keywords; color Doppler, uterine artery embolization, expectant management 16 1 1 Abstract 2 3 Cases of retained products of conception (RPOC) with marked vascularity present a 4 clinical challenge because simple dilation and curettage (D&C) can lead to 5 life-threatening hemorrhage. -

Operative Hysteroscopy for Retained Products of Conception: Efficacy and Subsequent Fertility
Journal of Gynecology Obstetrics and Human Reproduction 48 (2019) 151–154 Available online at ScienceDirect www.sciencedirect.com Original Article Operative hysteroscopy for retained products of conception: Efficacy and subsequent fertility a,b, a a a Perrine Capmas *, Anina Lobersztajn , Laura Duminil , Tiphaine Barral , a,c a,b,c Anne-Gaëlle Pourcelot , Hervé Fernandez a AP-HP, Department of Gynecology and Obstetrics, Hospital Bicêtre, GHU Sud, F-94276, Le Kremlin Bicêtre, France b INSERM, U1018, Centre of research in Epidemiology and population health (CESP), F-94276, Le Kremlin Bicêtre, France c Faculty of medicine, University Paris Saclay, F-94276, Le Kremlin Bicêtre, France A R T I C L E I N F O A B S T R A C T Article history: Retained product of conception complicates nearly 1% of pregnancies and can lead to synechiae and Received 18 June 2018 compromise ulterior fertility. The aim of this study is to evaluate efficiency of operative hysteroscopy in Received in revised form 11 December 2018 management of retained products of conception (RPOC). Secondary objectives are assessments of intra- Accepted 12 December 2018 uterine adhesions rate and later fertility. Available online 12 December 2018 This unicentric retrospective study includes women who undertook an operative hysteroscopy for retained products of conception between January 2012 and March 2014. Assessment of the efficiency of Keywords: operative hysteroscopy is defined by a complete resection of retained products of conception confirmed Hysteroscopy by office hysteroscopy. Retained product of conception One hundred fourteen women were included in the study. Efficiency of operative hysteroscopy for Intra-uterine adhesions Fertility retained products of conception is 91% for women with a postoperative office hysteroscopy. -
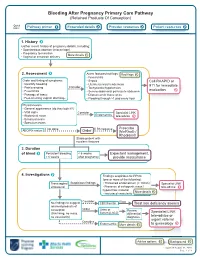
Bleeding After Pregnancy Primary Care Pathway (Retained Products of Conception)
Bleeding After Pregnancy Primary Care Pathway (Retained Products Of Conception) Quick links: Pathway primer Expanded details Provider resources Patient resources 1. History Gather recent history of pregnancy details, including: • Spontaneous abortion (miscarriage) • Pregnancy termination More details • Vaginal or cesarean delivery 2. Assessment Alarm features/red flags: Red flags • Fever/chills Order and timing of symptoms: • Sepsis Call RAAPID or • Quantify bleeding • Uterine/cervical tenderness 911 for immediate • Pain/cramping Consider • Tachycardia/hypotension • Fever/chills • Severe abdominal pain/acute abdomen evaluation • Passage of tissue • Dilated cervix tissue at os • Foul-smelling vaginal discharge • Flooding through >1 pad every hour Physical exam: • General appearance (do they look ill?) • Vital signs Consider Specialist LINK • Abdominal exam Endometritis tele-advice • Bimanual exam • Speculum exam If not done If Rh negative Prescribe ABO/Rh status: Order WinRho® / RhoGam® Stable patient with no alarm features 3. Duration of bleed Persistent bleeding < 6 weeks Expectant management, > 6 weeks after pregnancy provide reassurance 4. Investigations Findings suspicious for RPOC (one or more of the following): Transvaginal Suspicious findings • Thickened endometrium (> 10mm) Specialist LINK ultrasound • Presence of echogenic mass / tele-advice hypoechoic material More details • Increased vascularity Consider If low No findings to suggest CBC/Ferritin Treat iron deficiency anemia retained products of conception Order Urine or Review Specialist LINK (thin lining, no mass, Serum β-hCG differential no vascularity) diagnosis tele-advice or urgent referral Consider Endometritis More details to gynecology Advice options Background Updated: August 31, 2020 Page 1 of 9 PATHWAY PRIMER A diagnosis of retained products of conception (RPOC) occurs when placental or pregnancy tissue persists in the uterus following spontaneous abortion (miscarriage), pregnancy termination, or vaginal or caesarean delivery. -

Dilatation & Evacuation (D&E) Reference Guide
Dilatation & Evacuation (D&E) Reference Guide INDUCED ABORTION AND POSTABORTION CARE AT OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) © 2017, 2018 Ipas ISBN: 1-933095-98-9 Citation: Edelman, A. & Kapp, N. (2018). Dilatation & Evacuation (D&E) Reference Guide: Induced abortion and postabortion care at or after 13 weeks gestation (‘second trimester’). Chapel Hill, NC: Ipas. Ipas works globally so that women and girls have improved sexual and reproductive health and rights through enhanced access to and use of safe abortion and contraceptive care. We believe in a world where every woman and girl has the right and ability to determine her own sexuality and reproductive health. Ipas is a registered 501(c)(3) nonprofit organization. All contributions to Ipas are tax deductible to the full extent allowed by law. For more information or to donate to Ipas: Ipas P.O. Box 9990 Chapel Hill, NC 27515 USA 1-919-967-7052 www.ipas.org Cover photo: © Richard Lord The photographs used in this publication are for illustrative purposes only; they do not imply any particular attitudes, behaviors, or actions on the part of any person who appears in the photographs. Printed on recycled paper. Dilatation & Evacuation (D&E) Reference Guide: INDUCED ABORTION AND POSTABORTION CARE AT OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) Alison Edelman Senior Clinical Consultant, Ipas Professor, OB/GYN Oregon Health & Science University Nathalie Kapp Associate Medical Director, Ipas MD, MPH About Ipas Ipas works globally so that women and girls have improved sexual and reproductive health and rights through enhanced access to and use of safe abortion and contraceptive care. -

Surgical Management of Miscarriage and Removal of Persistent Placental Or Fetal Remains
Surgical Management of Miscarriage and Removal of Persistent Placental or Fetal Remains Consen t Advice No. 10 (Joint with AEPU) January 2018 Surgical Management of Miscarriage and Removal of Persistent Placental or Fetal Remains This is the second edition of this guidance, which was published in 2010 under the title Surgical Evacuation of the Uterus for Early Pregnancy Loss . This paper provides advice for health professionals obtaining consent from women undergoing surgical management of miscarriage with electric or manual vacuum aspiration. It is also intended to be appropriate when surgical intervention is indicated for an incomplete termination of pregnancy, incomplete or delayed miscarriage, or partially retained placenta after delivery. After careful discussion with the woman, the consent form should be edited under the heading ‘Name of proposed procedure or course of treatment’ to accurately describe the exact procedure to be performed. The paper follows the structure of Consent Form 1 from the Department of Health, England 1/Welsh Assembly Government 2/Department of Health, Social Services and Public Safety, Northern Ireland. 3 It should be used as part of the consent process outlined in the Royal College of Obstetricians and Gynaecologists (RCOG) website at rcog.org.uk/consent, and in conjunction with the RCOG Clinical Governance Advice No. 6 Obtaining Valid Consent 4 and Clinical Governance Advice No. 7 Presenting Information on Risk. 5 Please also refer to the National Institute for Health and Care Excellence (NICE) clinical guideline 154 Ectopic pregnancy and miscarriage: diagnosis and initial management. 6 The aim of this advice is to ensure that all women are given consistent and adequate information for consent. -

Overview of Neo-Vascular Lesions After Delivery Or Miscarriage
Journal of Clinical Medicine Review Overview of Neo-Vascular Lesions after Delivery or Miscarriage Yuji Shiina Department of Obstetrics and Gynecology, Yamagata Prefectural Shinjo Hospital, Shinjo, 12-55 Wakabacho, Yamagata 996-0025, Japan; [email protected]; Tel.: +81-233-22-5525; Fax: +81-233-29-2693 Abstract: The concept of intrauterine neo-vascular lesions after pregnancy, initially called placental polyps, has changed gradually. Now, based on diagnostic imaging, such lesions are defined as retained products of conception (RPOC) with vascularization. The lesions appear after delivery or miscarriage, and they are accompanied by frequent abundant vascularization in the myometrium attached to the remnant. Many of these vascular lesions have been reported to resolve spontaneously within a few months. Acquired arteriovenous malformations (AVMs) must be considered in the differential diagnosis of RPOC with vascularization. AVMs are errors of morphogenesis. The lesions start to be constructed at the time of placenta formation. These lesions do not show spontaneous regression. Although these two lesions are recognized as neo-vascular lesions, neo-vascular lesions on imaging may represent conditions other than these two lesions (e.g., peritrophoblastic flow, uterine artery pseudoaneurysm, and villous-derived malignancies). Detecting vasculature at the placenta–myometrium interface and classifying vascular diseases according to hemodynamics in the remnant would facilitate the development of specific treatments. Keywords: placental polyps; retained products of conception; arteriovenous malformations; neo- vascular lesion Citation: Shiina, Y. Overview of Neo-Vascular Lesions after Delivery or Miscarriage. J. Clin. Med. 2021, 10, 1084. https://doi.org/10.3390/ 1. Introduction jcm10051084 The sudden onset of critical vaginal bleeding 12 years after pregnancy was reported in 1884 in Philadelphia [1].