Oh Baby! OB Coding for ICD-10-PCS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
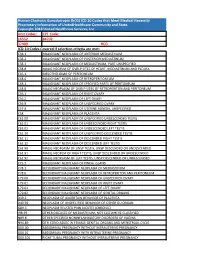
Human Chorionic Gonadotropin (Hcg) ICD 10 Codes That Meet Medical Necessity Proprietary Information of Unitedhealthcare Community and State
Human Chorionic Gonadotropin (hCG) ICD 10 Codes that Meet Medical Necessity Proprietary Information of UnitedHealthcare Community and State. Copyright 2018 United Healthcare Services, Inc Unit Codes: CPT Code: 16552 84702 37409 HCG ICD-10 Codes Covered if selection criteria are met: C38.1 MALIGNANT NEOPLASM OF ANTERIOR MEDIASTINUM C38.2 MALIGNANT NEOPLASM OF POSTERIOR MEDIASTINUM C38.3 MALIGNANT NEOPLASM OF MEDIASTINUM, PART UNSPECIFIED C38.8 MALIG NEOPLM OF OVRLP SITES OF HEART, MEDIASTINUM AND PLEURA C45.1 MESOTHELIOMA OF PERITONEUM C48.0 MALIGNANT NEOPLASM OF RETROPERITONEUM C48.1 MALIGNANT NEOPLASM OF SPECIFIED PARTS OF PERITONEUM C48.8 MALIG NEOPLASM OF OVRLP SITES OF RETROPERITON AND PERITONEUM C56.1 MALIGNANT NEOPLASM OF RIGHT OVARY C56.2 MALIGNANT NEOPLASM OF LEFT OVARY C56.9 MALIGNANT NEOPLASM OF UNSPECIFIED OVARY C57.4 MALIGNANT NEOPLASM OF UTERINE ADNEXA, UNSPECIFIED C58 MALIGNANT NEOPLASM OF PLACENTA C62.00 MALIGNANT NEOPLASM OF UNSPECIFIED UNDESCENDED TESTIS C62.01 MALIGNANT NEOPLASM OF UNDESCENDED RIGHT TESTIS C62.02 MALIGNANT NEOPLASM OF UNDESCENDED LEFT TESTIS C62.10 MALIGNANT NEOPLASM OF UNSPECIFIED DESCENDED TESTIS C62.11 MALIGNANT NEOPLASM OF DESCENDED RIGHT TESTIS C62.12 MALIGNANT NEOPLASM OF DESCENDED LEFT TESTIS C62.90 MALIG NEOPLASM OF UNSP TESTIS, UNSP DESCENDED OR UNDESCENDED C62.91 MALIG NEOPLM OF RIGHT TESTIS, UNSP DESCENDED OR UNDESCENDED C62.92 MALIG NEOPLASM OF LEFT TESTIS, UNSP DESCENDED OR UNDESCENDED C75.3 MALIGNANT NEOPLASM OF PINEAL GLAND C78.1 SECONDARY MALIGNANT NEOPLASM OF MEDIASTINUM C78.6 SECONDARY -

Refusal to Undergo a Cesarean Section: a Woman's Right Or a Criminal Act ? Monica K
Health Matrix: The Journal of Law- Medicine Volume 15 | Issue 2 2005 Refusal to Undergo a Cesarean Section: A Woman's Right or a Criminal Act ? Monica K. Miller Follow this and additional works at: https://scholarlycommons.law.case.edu/healthmatrix Part of the Health Law and Policy Commons Recommended Citation Monica K. Miller, Refusal to Undergo a Cesarean Section: A Woman's Right or a Criminal Act ?, 15 Health Matrix 383 (2005) Available at: https://scholarlycommons.law.case.edu/healthmatrix/vol15/iss2/6 This Article is brought to you for free and open access by the Student Journals at Case Western Reserve University School of Law Scholarly Commons. It has been accepted for inclusion in Health Matrix: The ourJ nal of Law-Medicine by an authorized administrator of Case Western Reserve University School of Law Scholarly Commons. REFUSAL TO UNDERGO A CESAREAN SECTION: A WOMAN'S RIGHT OR A CRIMINAL ACT? Monica K. Millert INTRODUCTION In March, 2004, Melissa Ann Rowland, a twenty-eight-year-old woman from Salt Lake City, gained national media attention when she was arrested on charges of homicide relating to the death of her son. Although there are many child homicide cases that occur regularly across the country that do not attract wide-spread media attention, this case was exceptional because her son died before he was ever born. 1 Rowland had sought medical treatment several times between late December 2003 and January 9, 2004. Each time, she was allegedly advised to get immediate medical treatment, including a cesarean sec- tion (c-section), because her twin fetuses were in danger of death or serious injury. -

1063 Relation Between Vaginal Hiatus and Perineal Body
1063 Campanholi V1, Sanches M1, Zanetti M R D1, Alexandre S1, Resende A P M1, Petricelli C D1, Nakamura M U1 1. Unifesp- Brasil RELATION BETWEEN VAGINAL HIATUS AND PERINEAL BODY LENGTHS WITH EPISIOTOMY IN VAGINAL DELIVERY Hypothesis / aims of study The aim of the study was to assess the relationship between vaginal hiatus and perineal body lengths with the occurrence of episiotomy during vaginal delivery. Study design, materials and methods It´s a cross-sectional observational study with a consecutive sample of 60 parturients, made from July 2009 to March 2010 in the Obstetric Center at University Hospital in São Paulo, Brazil. Inclusion criteria were parturients at term (37 to 42 weeks gestation) in the first stage of labour, with less than 9 cm dilatation, with a single fetus in cephalic presentation and good vitality confirmed by cardiotocography. Exclusion criteria were parturients submitted to cesarean section or forceps delivery. The patients were evaluated in the lithotomic position. The measurement was performed in the first stage of labour, by the same examiner using a metric measuring tape previously cleaned with alcohol 70% and discarded after each use. The vaginal hiatus length (distance between the external urethral meatus and the vulvar fourchette) and the perineal body (distance between the vulvar fourchette and the center of the anal orifice) were evaluated. For statistical analysis the SPSS (Statistical Package for Social Sciences) version 17® was used, applying Mann-Whitney Test and Spearman Rank Correlation Test to determine the importance of vaginal hiatus and perineal body length in the occurrence of episiotomy, with p<0.05. -

Clinical Policy: Fetal Surgery in Utero for Prenatally Diagnosed
Clinical Policy: Fetal Surgery in Utero for Prenatally Diagnosed Malformations Reference Number: CP.MP.129 Effective Date: 01/18 Coding Implications Last Review Date: 09/18 Revision Log Description This policy describes the medical necessity requirements for performing fetal surgery. This becomes an option when it is predicted that the fetus will not live long enough to survive delivery or after birth. Therefore, surgical intervention during pregnancy on the fetus is meant to correct problems that would be too advanced to correct after birth. Policy/Criteria I. It is the policy of Pennsylvania Health and Wellness® (PHW) that in-utero fetal surgery (IUFS) is medically necessary for any of the following: A. Sacrococcygeal teratoma (SCT) associated with fetal hydrops related to high output heart failure : SCT resecton: B. Lower urinary tract obstruction without multiple fetal abnormalities or chromosomal abnormalities: urinary decompression via vesico-amniotic shunting C. Ccongenital pulmonary airway malformation (CPAM) and extralobar bronchopulmonary sequestration with hydrops (hydrops fetalis): resection of malformed pulmonary tissue, or placement of a thoraco-amniotic shunt; D. Twin-twin transfusion syndrome (TTTS): treatment approach is dependent on Quintero stage, maternal signs and symptoms, gestational age and the availability of requisite technical expertise and include either: 1. Amnioreduction; or 2. Fetoscopic laser ablation, with or without amnioreduction when member is between 16 and 26 weeks gestation; E. Twin-reversed-arterial-perfusion (TRAP): ablation of anastomotic vessels of the acardiac twin (laser, radiofrequency ablation); F. Myelomeningocele repair when all of the following criteria are met: 1. Singleton pregnancy; 2. Upper boundary of myelomeningocele located between T1 and S1; 3. -

Delivery Mode for Prolonged, Obstructed Labour Resulting in Obstetric Fistula: a Etrr Ospective Review of 4396 Women in East and Central Africa
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by eCommons@AKU eCommons@AKU Obstetrics and Gynaecology, East Africa Medical College, East Africa 12-17-2019 Delivery mode for prolonged, obstructed labour resulting in obstetric fistula: a etrr ospective review of 4396 women in East and Central Africa C. J. Ngongo T. J. Raassen L. Lombard J van Roosmalen S. Weyers See next page for additional authors Follow this and additional works at: https://ecommons.aku.edu/eastafrica_fhs_mc_obstet_gynaecol Part of the Obstetrics and Gynecology Commons Authors C. J. Ngongo, T. J. Raassen, L. Lombard, J van Roosmalen, S. Weyers, and Marleen Temmerman DOI: 10.1111/1471-0528.16047 www.bjog.org Delivery mode for prolonged, obstructed labour resulting in obstetric fistula: a retrospective review of 4396 women in East and Central Africa CJ Ngongo,a TJIP Raassen,b L Lombard,c J van Roosmalen,d,e S Weyers,f M Temmermang,h a RTI International, Seattle, WA, USA b Nairobi, Kenya c Cape Town, South Africa d Athena Institute VU University Amsterdam, Amsterdam, The Netherlands e Leiden University Medical Centre, Leiden, The Netherlands f Department of Obstetrics and Gynaecology, Ghent University Hospital, Ghent, Belgium g Centre of Excellence in Women and Child Health, Aga Khan University, Nairobi, Kenya h Faculty of Medicine and Health Science, Ghent University, Ghent, Belgium Correspondence: CJ Ngongo, RTI International, 119 S Main Street, Suite 220, Seattle, WA 98104, USA. Email: [email protected] Accepted 3 December 2019. Objective To evaluate the mode of delivery and stillbirth rates increase occurred at the expense of assisted vaginal delivery over time among women with obstetric fistula. -

Fetal Surgery in Utero for Prenatally Diagnosed Malformations
Clinical Policy: Fetal Surgery in Utero for Prenatally Diagnosed Malformations Reference Number: PA.CP.MP.129 Effective Date: 01/18 Coding Implications Last Review Date: 12/18 Revision Log Description This policy describes the medical necessity requirements for performing fetal surgery. This becomes an option when it is predicted that the fetus will not live long enough to survive delivery or after birth. Therefore, surgical intervention during pregnancy on the fetus is meant to correct problems that would be too advanced to correct after birth. Policy/Criteria I. It is the policy of Pennsylvania Health and Wellness® (PHW) that in-utero fetal surgery (IUFS) is medically necessary for any of the following: A. Sacrococcygeal teratoma (SCT) associated with fetal hydrops related to high output heart failure : SCT resection; B. Lower urinary tract obstruction without multiple fetal abnormalities or chromosomal abnormalities: urinary decompression via vesico-amniotic shunting C. Congenital pulmonary airway malformation (CPAM) and extralobar bronchopulmonary sequestration with hydrops (hydrops fetalis): resection of malformed pulmonary tissue, or placement of a thoraco-amniotic shunt; D. Twin-twin transfusion syndrome (TTTS): treatment approach is dependent on Quintero stage, maternal signs and symptoms, gestational age and the availability of requisite technical expertise and include either: 1. Amnioreduction; or 2. Fetoscopic laser ablation, with or without amnioreduction when member is between 16 and 26 weeks gestation; E. Twin-reversed-arterial-perfusion (TRAP): ablation of anastomotic vessels of the acardiac twin (laser, radiofrequency ablation); F. Myelomeningocele repair when all of the following criteria are met: 1. Singleton pregnancy; 2. Upper boundary of myelomeningocele located between T1 and S1; 3. -

Histomorphological Study of Chorionic Villi in Products of Conception Following First Trimester Abortions
November, 2018/ Vol 4/ Issue 7 Print ISSN: 2456-9887, Online ISSN: 2456-1487 Original Research Article Histomorphological study of chorionic villi in products of conception following first trimester abortions Shilpa MD1, Supreetha MS2, Varshashree3 1Dr. Shilpa, MD, Assistant Professor, 2Dr. Supreetha, MS, Assistant Professor, 3Dr. Varshashree, Post graduate, all authors are affiliated with Department of Pathology, Sri Devaraj Urs Medical College, Tamaka, Kolar, Karnataka, India. Corresponding Author: Dr. Shilpa, MD, Assistant Professor, Department of Pathology, Sri Devaraj Urs Medical College, Tamaka, Kolar, Karnataka, India. Email id: [email protected] ………………………………………………………………………………………………………………………………... Abstract Background: The common problem which occurs in first trimester of pregnancy is miscarriage. Retained products of conception are commonly received specimen for histopathological examination. Apart from confirmation of pregnancy, a careful examination can provide some additional information about the cause or the conditions associated with abortion. Aim: 1. To study various histopathological changes occurring in chorionic villi in first trimester spontaneous abortions and to know the pathogenesis of abortions. Materials and methods: This was a cross sectional retrospective study carried out for over a period of 3 years from January 2015 to January 2018. A total of 235biopsies were obtained from patient with the diagnosis of the first trimester spontaneous abortions were included in this study. Results: In our study most common age group of the abortion was between 21-30 years (63%). Incomplete abortion was the commonest type of abortion (47.7%). Many dysmorphic features were observed in this study like hydropic change (67%), stromal fibrosis (62%), villi with reduced blood vessels (52.7%) and perivillous fibrin deposition. -

Medical Abortion Reference Guide INDUCED ABORTION and POSTABORTION CARE at OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) © 2017, 2018 Ipas
Medical Abortion Reference Guide INDUCED ABORTION AND POSTABORTION CARE AT OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) © 2017, 2018 Ipas ISBN: 1-933095-97-0 Citation: Edelman, A. & Mark, A. (2018). Medical Abortion Reference Guide: Induced abortion and postabortion care at or after 13 weeks gestation (‘second trimester’). Chapel Hill, NC: Ipas. Ipas works globally so that women and girls have improved sexual and reproductive health and rights through enhanced access to and use of safe abortion and contraceptive care. We believe in a world where every woman and girl has the right and ability to determine her own sexuality and reproductive health. Ipas is a registered 501(c)(3) nonprofit organization. All contributions to Ipas are tax deductible to the full extent allowed by law. For more information or to donate to Ipas: Ipas P.O. Box 9990 Chapel Hill, NC 27515 USA 1-919-967-7052 [email protected] www.ipas.org Cover photo: © Ipas The photographs used in this publication are for illustrative purposes only; they do not imply any particular attitudes, behaviors, or actions on the part of any person who appears in the photographs. Printed on recycled paper. Medical Abortion Reference Guide INDUCED ABORTION AND POSTABORTION CARE AT OR AFTER 13 WEEKS GESTATION (‘SECOND TRIMESTER’) Alison Edelman Senior Clinical Consultant, Ipas Professor, OB/GYN Oregon Health & Science University Alice Mark Associate Medical Director National Abortion Federation About Ipas Ipas works globally so that women and girls have improved sexual and reproductive health and rights through enhanced access to and use of safe abortion and contraceptive care. -

Miscarriage Or Early Pregnancy Loss- Diagnosis and Management (Version 5)
Miscarriage or early pregnancy loss- diagnosis and management (Version 5) Guideline Readership This guideline applies to all women diagnosed with miscarriage in early pregnancy (up to 13 completed weeks) within the Heart of England Foundation Trust and to attending clinicians, sonographers and nursing staff on Gynaecology ward and early pregnancy unit. All care is tailored to individual patient needs, with an in-depth discussion of the intended risks and benefits for any intervention offered to woman with early pregnancy loss. Guideline Objectives The objective of this guideline is to enable all clinicians to recognise the different types of miscarriages and to follow a recognised management pathway so that all women with actual or suspected miscarriage receive, an appropriate and individualised care. Other Guidance Ectopic pregnancy and miscarriage: diagnosis and initial management. NICE guidance Dec 2012 Ratified Date: Insert Date Launch Date: 16 March 2018 Review Date: 16 March 2021 Guideline Author: Dr Rajmohan, Dr Cheema Contents & page numbers: 1. Flowcharts Flowchart 1 – Management of complete miscarriage p3 Flowchart 2 – Management of incomplete miscarriage p4 Flowchart 3 – Management of Missed miscarriage p5 Flowchart 4 - Management of Early fetal demise p6 Flowchart 5 – Medical management of miscarriage p7 Flowchart 6 - Surgical management (SMM) pathway p8 2. Executive summary and Overview p9 3. Body of guideline Types of miscarriage p9 Threatened miscarriage p9 Complete miscarriage p9 Incomplete miscarriage p9 Missed miscarriage -

A Guide to Obstetrical Coding Production of This Document Is Made Possible by Financial Contributions from Health Canada and Provincial and Territorial Governments
ICD-10-CA | CCI A Guide to Obstetrical Coding Production of this document is made possible by financial contributions from Health Canada and provincial and territorial governments. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. Unless otherwise indicated, this product uses data provided by Canada’s provinces and territories. All rights reserved. The contents of this publication may be reproduced unaltered, in whole or in part and by any means, solely for non-commercial purposes, provided that the Canadian Institute for Health Information is properly and fully acknowledged as the copyright owner. Any reproduction or use of this publication or its contents for any commercial purpose requires the prior written authorization of the Canadian Institute for Health Information. Reproduction or use that suggests endorsement by, or affiliation with, the Canadian Institute for Health Information is prohibited. For permission or information, please contact CIHI: Canadian Institute for Health Information 495 Richmond Road, Suite 600 Ottawa, Ontario K2A 4H6 Phone: 613-241-7860 Fax: 613-241-8120 www.cihi.ca [email protected] © 2018 Canadian Institute for Health Information Cette publication est aussi disponible en français sous le titre Guide de codification des données en obstétrique. Table of contents About CIHI ................................................................................................................................. 6 Chapter 1: Introduction .............................................................................................................. -

Clinical and Physical Aspects of Obstetric Vacuum Extraction
CLINICAL AND PHYSICAL ASPECTS OF OBSTETRIC VACUUM EXTRACTION KLINISCHE EN FYSISCHE ASPECTEN VAN OnSTETRISCHE VACUUM EXTRACTIE Clinical and physical aspects of obstetric vacuum extraction I Jette A. Kuit Thesis Rotterdam - with ref. - with summary in Dutch ISBN 90-9010352-X Keywords Obstetric vacuum extraction, oblique traction, rigid cup, pliable cup, fetal complications, neonatal retinal hemorrhage, forceps delivery Copyright Jette A. Kuit, Rotterdam, 1997. All rights reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the holder of the copyright. Cover, and drawings in the thesis, by the author. CLINICAL AND PHYSICAL ASPECTS OF OBSTETRIC VACUUM EXTRACTION KLINISCHE EN FYSISCHE ASPECTEN VAN OBSTETRISCHE VACUUM EXTRACTIE PROEFSCHRIFf TER VERKRUGING VAN DE GRAAD VAN DOCTOR AAN DE ERASMUS UNIVERSITEIT ROTTERDAM OP GEZAG VAN DE RECTOR MAGNIFICUS PROF. DR. P.W.C. AKKERMANS M.A. EN VOLGENS BESLUIT VAN HET COLLEGE VOOR PROMOTIES DE OPENBARE VERDEDIGING ZAL PLAATSVINDEN OP WOENSDAG 2 APRIL 1997 OM 15.45 UUR DOOR JETTE ALBERT KUIT GEBOREN TE APELDOORN Promotiecommissie Promotor Prof.dr. H.C.S. Wallenburg Overige leden Prof.dr. A.C. Drogendijk Prof. dr. G.G.M. Essed Prof.dr.ir. C.l. Snijders Co-promotor Dr. F.J.M. Huikeshoven To my parents, to Irma, Suze and Julius. CONTENTS 1. GENERAL INTRODUCTION 9 2. VACUUM CUPS AND VACUUM EXTRACTION; A REVIEW 13 2.1. Introduction 2.2. Obstetric vacuum cups in past and present 2.2.1. Historical backgroulld 2.2.2. -

Leapfrog Hospital Survey Hard Copy
Leapfrog Hospital Survey Hard Copy QUESTIONS & REPORTING PERIODS ENDNOTES MEASURE SPECIFICATIONS FAQS Table of Contents Welcome to the 2016 Leapfrog Hospital Survey........................................................................................... 6 Important Notes about the 2016 Survey ............................................................................................ 6 Overview of the 2016 Leapfrog Hospital Survey ................................................................................ 7 Pre-Submission Checklist .................................................................................................................. 9 Instructions for Submitting a Leapfrog Hospital Survey ................................................................... 10 Helpful Tips for Verifying Submission ......................................................................................... 11 Tips for updating or correcting a previously submitted Leapfrog Hospital Survey ...................... 11 Deadlines ......................................................................................................................................... 13 Deadlines for the 2016 Leapfrog Hospital Survey ...................................................................... 13 Deadlines Related to the Hospital Safety Score ......................................................................... 13 Technical Assistance.......................................................................................................................