The Green Fluorescent Protein: Discovery, Expression and Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acidic Phospholipids,47, 58 Acidification Steps, 23 Adsorbent, 53
Index acidic phospholipids,47, 58 ATPase, 22, 107 acidification steps, 23 auto-oxidation, 48,49 adsorbent, 53 aequorin, 95 aggregated mitochondria, 23 bacterial cells, 10 alcohols, 49 bacteriorhodopsin (BR), 218,232 alkaline hydrolysis, I bee venom, 121 alkyl esters, 57, 59 binders,53 amide bond, 129 silica gel, 53, 65, 176 amide linkage, 14 silica gel H, 176 amino alcohol, II bioactive phospholipids, 144 amino-group labelling reagents, 121 blanching, 50 aminopeptidase, 17 buoyant density, 16 aminophospholipid, 113, 118 butyl hydroxy toluene (BHT), 49,212 aminophospholipid pump, 119, 140 aminophospholipid translocase, 11 3 amphotericin B (AmB), 107 Cal+ -ATPases, 28, 107 N-(1-deoxyD-fructos-1-yl) AmB, 107 Cal+ -uptake, 27 anchored lipids, 38 campesterol, 104 angular amplitude, 89 calciferol, 78 anilino-8-naphthalene sulfonate (ANS), Candida albicans, 59, 60, 61, 76, 78, 102, 94, 103 104, 108 animal cells, 10 caproic acid, 129, 130 animal tissues, 9 carotenoids, 37 anion transporter, 135 cell debris, 33 anisotropy, 81 , 89, 95, 96, 97, 102, 104, cell disintegrator, 19 105 ceramide, 14, 129, 194 antagonists, 178 ceramide monohexosides ( CMH), 60 antioxidant, 49, 50 cerebroside, 14 apolar lipids, 56, 57, 69 chemical probes,112 arachidonic acid (AA), 144,153, 161 chloroplast, 32, 33 artificial membrane, 83 isolation, 32, 33 ascending chromatography, 54 purification, 33 asymmetric topology, 128 cholesterol, 14, 15,212 asymmetry, 112, 113,119 choline, 7, 9 atebrin, 95 chromatograms, 55 Index 249 chromatographic analyses, 52 ELISA, 146, 148 -

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -

Bioluminescent Properties of Semi-Synthetic Obelin and Aequorin Activated by Coelenterazine Analogues with Modifications of C-2, C-6, and C-8 Substituents
International Journal of Molecular Sciences Article Bioluminescent Properties of Semi-Synthetic Obelin and Aequorin Activated by Coelenterazine Analogues with Modifications of C-2, C-6, and C-8 Substituents 1, 2,3, 1 2, Elena V. Eremeeva y , Tianyu Jiang y , Natalia P. Malikova , Minyong Li * and Eugene S. Vysotski 1,* 1 Photobiology Laboratory, Institute of Biophysics SB RAS, Federal Research Center “Krasnoyarsk Science Center SB RAS”, Krasnoyarsk 660036, Russia; [email protected] (E.V.E.); [email protected] (N.P.M.) 2 Key Laboratory of Chemical Biology (MOE), Department of Medicinal Chemistry, School of Pharmaceutical Sciences, Shandong University, Jinan 250012, China; [email protected] 3 State Key Laboratory of Microbial Technology, Shandong University–Helmholtz Institute of Biotechnology, Shandong University, Qingdao 266237, China * Correspondence: [email protected] (M.L.); [email protected] (E.S.V.); Tel.: +86-531-8838-2076 (M.L.); +7-(391)-249-44-30 (E.S.V.); Fax: +86-531-8838-2076 (M.L.); +7-(391)-290-54-90 (E.S.V.) These authors contributed equally to this work. y Received: 23 June 2020; Accepted: 27 July 2020; Published: 30 July 2020 Abstract: Ca2+-regulated photoproteins responsible for bioluminescence of a variety of marine organisms are single-chain globular proteins within the inner cavity of which the oxygenated coelenterazine, 2-hydroperoxycoelenterazine, is tightly bound. Alongside with native coelenterazine, photoproteins can also use its synthetic analogues as substrates to produce flash-type bioluminescence. However, information on the effect of modifications of various groups of coelenterazine and amino acid environment of the protein active site on the bioluminescent properties of the corresponding semi-synthetic photoproteins is fragmentary and often controversial. -

GFP: Lighting up Life
PERSPECTIVE GFP: Lighting up life Martin Chalfie1 Department of Biological Sciences, Columbia University, New York, NY 10027 You can observe a lot by watching. Zernike, physics, 1953), large-array ra- My colleagues and I often call their Yogi Berra dio telescopes (Martin Ryle, physics, Nobel Prize the first worm prize. The 1974), the electron microscope (Ernst second went in 2006 to Andy Fire and My companions and I then witnessed Ruska, physics, 1986), the scanning tun- Craig Mello for their discovery of RNA a curious spectacle...TheNautilus neling microscope (Gerd Binnig and interference. I consider this year’s prize floated in the midst of ...trulyliv- Heinrich Rohrer, physics, 1986), com- to be the third worm prize, because if I ing light[,]...aninfinite agglomera- puter-assisted tomography (Allan M. had not worked on C. elegans and con- tion of colored...globules of diaph- Cormack and Godfrey N. Hounsfield, stantly told people that one of its advan- anous jelly.... physiology or medicine, 1979), and, tages was that it was transparent, I am Jules Verne, Twenty Thousand most recently, magnetic resonance imag- convinced I would have ignored GFP Leagues Under the Sea ing (Paul C. Lauterbur and Sir Peter when I first heard of it. These three Now it is such a bizarrely improbable Mansfield, physiology or medicine, prizes speak to the genius of Sydney coincidence that anything so mind- 2003). Brenner in choosing and developing a bogglingly useful could have evolved My road to imaging was not direct. I new organism for biological research. purely by chance that some thinkers had been interested in science from The year before I learned about GFP, have chosen to see it as a final and when I was very young, but after a di- my lab had begun looking at gene expres- clinching proof of the nonexistence sastrous summer lab experience in which sion in the C. -

Bioluminescence Related Publications from JNC Corporation
Bioluminescence related publications since 1985 110) Inouye, S. and Hojo, H. (2018) Revalidation of recombinant aequorin as a light emission standard: Estimation of specific activity of Gaussia luciferase. Biochem. Biophys. Res. Commun. 507: 242-245. 109) Yokawa, S., Suzuki, T., Hayashi, A., Inouye, S., Inoh, Y. and Furuno, T. (2018) Video-rate bioluminescence imaging of degranulation of mast cells attached to the extracellular matrix. Front. Cell Dev. Biol. 6: 74. 108) Inouye, S., Tomabechi, Y., Hosoya, T., Sekine, S. and Shirouzu, M. (2018) Slow luminescence kinetics of semi-synthetic aequorin:expression, purification and structure determination of cf3-aequorin. J. Biochem. 164(3): 247–255. 107) Inouye, S. (2018) Single-step purification of recombinant Gaussia luciferase from serum-containing culture medium of mammalian cells. Protein Expr. Purif. 141: 32-38. 106) Inouye, S. and Sahara-Miura, Y. (2017) A fusion protein of the synthetic IgG-binding domain and aequorin: Expression and purification from E. coli cells and its application. Protein Expr. Purif. 137: 58-63. 105) Suzuki, T., Kanamori, T. and Inouye, S. (2017) Quantitative visualization of synchronized insulin secretion from 3D-cultured cells. Biochem. Biophys. Res. Commun. 486: 886-892. 104) Yokawa, S., Suzuki, T., Inouye, S., Inoh, Y., Suzuki, R., Kanamori, T., Furuno, T. and Hirashima, N. (2017) Visualization of glucagon secretion from pancreatic α cells by bioluminescence video microscopy: Identification of secretion sites in the intercellular contact regions. Biochem. Biophys. Res. Commun. 485: 725-730. 103) Inouye, S. and Suzuki, T. (2016) Protein expression of preferred human codon-optimized Gaussia luciferase genes with an artificial open-reading frame in mammalian and bacterial cells. -
Poster Final Copy
Introduction to Jellyfish Jellyfish have been around for over 700 million years making them one of the oldest living creatures on earth. There are almost 3,000 species of jellyfish found throughout the world's oceans. From Cnidarian jellyfish, which are often referred as “true” jellyfish, to Ctenophores (comb jellyfish), Cubic Aquarium Systems have compiled this poster showing some of the most interesting species found around the globe. Moon Jellyfish Amakusa Jellyfish Mauve Stinger Purple Striped Jellyfish Japanese Sea Nettle Aurelia aurita Sanderia malayensis Pelagia noctilca Chrysaora colorata Chrysaora pacifica Pacific Sea Nettle Black Sea Nettle Lion’s Mane Jellyfish Blue Fire Jellyfish Egg Yolk Jellyfish Chrysaora fuscescens Chrysaora achlyos Cyanea capillata Cyanea lamarckii Phacellophora camtschatica Flame Jellyfish Blue Blubber Spotted Lagoon Jellyfish Australian Spotted Lagoon Jellyfish Upside Down Jellyfish Rhopilema esculentum Catostylus mosaicus Mastigias papu Phyllorhiza punctata Cassioppea sp. Mediterranean Jellyfish Crown Jellyfish Purple Jellyfish Chrystal Jellyfish Flower Hat Jellyfish Cotylorhiza tuberculata Cephea cephea Thysanostoma thysanura Aequorea victoria Olindias formosa Immortal Jellyfish Portuguese Man O’ War Box Jellyfish Comb Jellyfish Sea Gooseberry Turritopsis dohrni Physalia physali Chironex sp. Bolinopsis sp. Pleurobrachia bachei Cubic Aquarium Systems Exotic Aquaculture Jellyfish aquariums and specialised fish tanks | Cubic Aquarium Syste International Jellyfish Wholesale Cubic Aquarium Systems build a range of unique, specialised aquariums Exotic Aquaculture are a Hong Kong based aquarium livestock supplier for jellyfish and other unusual sea creatures specializing in jellyfish wholesale to the public and home aquarium trade. www.cubicaquarium.com Copyright Sanderia Group Limited www.exoticaquaculture.com All rights reserved. -

BPS Complete Catalog
BPSCATALOG ENZYMES KITS CELL LINES SCREENING SERVICES INNOVATIVE PRODUCTS TO FUEL YOUR EXPERIMENTS Unique, expert portfolio 2017 OUR MISSION: To provide the highest quality life science products and services worldwide in a timely manner to assist in accelerating drug discovery research and development for the treatment of human diseases. INNOVATION WE CONTINUOUSLY STRIVE TO BE FIRST TO MARKET BY PROVIDING EXPERTISE IN EXPRESSING HIGHLY ACTIVE ENZYMES MULTIPLE ASSAY DETECTION FORMATS HUMAN, MURINE AND MONKEY VERSIONS OF RECOMBINANT PROTEINS BIOTINYLATED VERSIONS OF MANY IMMUNE CHECKPOINT RECEPTORS 1ST AND MOST EXTENSIVE PARP ISOZYME PORTFOLIO 1ST AND MOST EXTENSIVE PDE ISOZYME PORTFOLIO 1ST COMPLETE SUITE OF HDAC AND SIRT ENZYMES 1ST AVAILABLE PARP AND TANKYRASE PROFILING SERVICES 1ST COMMERCIALLY AVAILABLE HISTONE DEMETHYLASES LARGEST OFFERING OF METHYLTRANSFERASES LARGEST OFFERING OF DEMETHYLASES OVER 200 PRODUCTS AND SERVICES EXCLUSIVE TO BPS RELIABILITY BPS IS CITED IN PEER-REVIEWED JOURNALS AND PUBLICATIONS AROUND THE WORLD NATURE GENETICS THE JOURNAL OF BIOLOGICAL CHEMISTRY JOURNAL OF CELL SCIENCE ANALYTICAL BIOCHEMISTRY BBRC NATURE CHEMICAL BIOLOGY ACS MEDICINAL CHEMISTRY LETTERS JOURNAL OF NATURAL PRODUCTS CHEMMEDCHEM MOLECULAR CANCER THERAPEUTICS & MANY MORE TABLE OF CONTENTS ACETYLTRANSFERASE 4 APOPTOSIS 4-5 ANTIBODIES 6-9 BIOTINYLATION 10-11 BROMODOMAINS 12-14 CELL BASED ASSAY KITS 15 CELL LINES 16-17 CELL SURFACE RECEPTORS 18-20 CYTOKINE(S) 21-24 DEACETYLASE(S) 25-27 DEMETHYLASE(S) 28-29 HEAT SHOCK PROTEINS 30 IMMUNOTHERAPY -

Ba3(P1−Xmnxo4)2 : Blue/Green Inorganic Materials Based on Tetrahedral Mn(V)
Bull. Mater. Sci., Vol. 34, No. 6, October 2011, pp. 1257–1262. c Indian Academy of Sciences. Ba3(P1−xMnxO4)2 : Blue/green inorganic materials based on tetrahedral Mn(V) SOURAV LAHA, ROHIT SHARMA, S V BHAT†, MLPREDDY‡, J GOPALAKRISHNAN∗ and S NATARAJAN Solid State and Structural Chemistry Unit, †Department of Physics, Indian Institute of Science, Bangalore 560 012, India ‡Chemical Science and Technology Division, National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695 019, India MS received 11 May 2011 ) 3− 2 Abstract. We describe a blue/green inorganic material, Ba3(P1−xMnxO4 2 (I) based on tetrahedral MnO4 :3d chromophore. The solid solutions (I) which are sky-blue and turquoise-blue for x ≤ 0·25 and dark green for x ≥ 0·50, 3− are readily synthesized in air from commonly available starting materials, stabilizing the MnO4 chromophore in an isostructural phosphate host. We suggest that the covalency/ionicity of P–O/Mn–O bonds in the solid solutions tunes the crystal field strength around Mn(V) such that a blue colour results for materials with small values of x. The material could serve as a nontoxic blue/green inorganic pigment. Keywords. Blue/green inorganic material; barium phosphate/manganate(V); tetrahedral manganate(V); blue/green chromophore; ligand field tuning of colour. 1. Introduction field transitions within an unusual five coordinated trigo- nal bipyramidal Mn(III). A turquoise-blue solid based on Inorganic solids displaying bright colours are important Li1·33Ti1·66O4 spinel oxide wherein the colour arises from as pigment materials which find a wide range of appli- an intervalence charge-transfer between Ti3+ and Ti4+ has cations in paints, inks, plastics, rubbers, ceramics, ena- also been described recently (Fernández-Osorio et al 2011). -

Nobel Lecture by Roger Y. Tsien
CONSTRUCTING AND EXPLOITING THE FLUORESCENT PROTEIN PAINTBOX Nobel Lecture, December 8, 2008 by Roger Y. Tsien Howard Hughes Medical Institute, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0647, USA. MOTIVATION My first exposure to visibly fluorescent proteins (FPs) was near the end of my time as a faculty member at the University of California, Berkeley. Prof. Alexander Glazer, a friend and colleague there, was the world’s expert on phycobiliproteins, the brilliantly colored and intensely fluorescent proteins that serve as light-harvesting antennae for the photosynthetic apparatus of blue-green algae or cyanobacteria. One day, probably around 1987–88, Glazer told me that his lab had cloned the gene for one of the phycobilipro- teins. Furthermore, he said, the apoprotein produced from this gene became fluorescent when mixed with its chromophore, a small molecule cofactor that could be extracted from dried cyanobacteria under conditions that cleaved its bond to the phycobiliprotein. I remember becoming very excited about the prospect that an arbitrary protein could be fluorescently tagged in situ by genetically fusing it to the phycobiliprotein, then administering the chromophore, which I hoped would be able to cross membranes and get inside cells. Unfortunately, Glazer’s lab then found out that the spontane- ous reaction between the apoprotein and the chromophore produced the “wrong” product, whose fluorescence was red-shifted and five-fold lower than that of the native phycobiliprotein1–3. An enzyme from the cyanobacteria was required to insert the chromophore correctly into the apoprotein. This en- zyme was a heterodimer of two gene products, so at least three cyanobacterial genes would have to be introduced into any other organism, not counting any gene products needed to synthesize the chromophore4. -
Color Vision Light
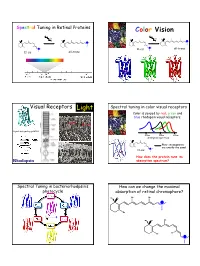
Spectral Tuning in Retinal Proteins Color Vision hν hν N N H H H H N all-trans N 11-cis 11-cis all-trans Visual Receptors Light Spectral tuning in color visual receptors Color is sensed by red, green and blue rhodopsin visual receptors. Rod Cone G-protein signaling pathway 400nm 500nm 600nm absorption spectrum Their chromophores H are exactly the same! 11-cis N How does the protein tune its Rhodopsin absorption spectrum? Spectral Tuning in bacteriorhodpsin’s How can we change the maximal photocycle absorption of retinal chromophore? Me Me Me Me N H Me Me Me Me N H Me Me Me Me Me Me Me H N 1 Excitation energy determines the Electronic Absorption maximal absorption Me Me Me Me π π* N - H S1 Me Absorption of light in the UV-VIS region of the S0 spectrum is due to Response excitation of electrons to higher energy levels. Me Me Me 13 7911 15 N H Me Me π-π* excitation in polyenes π-π* excitation in polyenes π∗ π∗ π∗ π∗ ∆E π∗ E photon E π π π π π Ground state (S0) Excited state (S1) ∆E (excitation energy, band gap) = hν = hc/λ blue-shift red-shift π-π* excitation in polyenes Tuning the length of the conjugated backbone β-carotene O O Vitamin A2 (retinal II) Vitamin A1 (retinal I) Longer wavelength Short wavelength 2 Retinal I Retinal II OPSIN SHIFT: how protein tunes the absorption maximum of its chromophore. Maximal absorption of protonated retinal Schiff base in: Water/methanol solution: 440 nm bR: 568 nm rod Rh: 500 nm red receptor: 560 nm green receptor: 530 nm blue receptor: 426 nm Salmon: different retinals in different stages of life Electrostatics and opsin shift Electrostatics and opsin shift S S positive charge 2 positive charge 2 S2 S2 Me Me Me Me Me Me S1 S1 S1 S1 + + + + N N H H Me O S0 Me O S0 Me O Me O C C Asp (Glu) Asp (Glu) S0 S0 no protein in protein no protein in protein counterion counterion • The counterion stabilizes the positive charge of the chromophore. -

Liberal Arts Science $600 Million in Support of Undergraduate Science Education
Janelia Update |||| Roger Tsien |||| Ask a Scientist SUMMER 2004 www.hhmi.org/bulletin LIBERAL ARTS SCIENCE In science and teaching— and preparing future investigators—liberal arts colleges earn an A+. C O N T E N T S Summer 2004 || Volume 17 Number 2 FEATURES 22 10 10 A Wellspring of Scientists [COVER STORY] When it comes to producing science Ph.D.s, liberal arts colleges are at the head of the class. By Christopher Connell 22 Cells Aglow Combining aesthetics with shrewd science, Roger Tsien found a bet- ter way to look at cells—and helped to revolutionize several scientif-ic disciplines. By Diana Steele 28 Night Science Like to take risks and tackle intractable problems? As construction motors on at Janelia Farm, the call is out for venturesome scientists with big research ideas. By Mary Beth Gardiner DEPARTMENTS 02 I N S T I T U T E N E W S HHMI Announces New 34 Investigator Competition | Undergraduate Science: $50 Million in New Grants 03 PRESIDENT’S LETTER The Scientific Apprenticeship U P F R O N T 04 New Discoveries Propel Stem Cell Research 06 Sleeper’s Hold on Science 08 Ask a Scientist 27 I N T E R V I E W Toward Détente on Stem Cell Research 33 G R A N T S Extending hhmi’s Global Outreach | Institute Awards Two Grants for Science Education Programs 34 INSTITUTE NEWS Bye-Bye Bio 101 NEWS & NOTES 36 Saving the Children 37 Six Antigens at a Time 38 The Emergence of Resistance 40 39 Hidden Potential 39 Remembering Santiago 40 Models and Mentors 41 Tracking the Transgenic Fly 42 Conduct Beyond Reproach 43 The 1918 Flu: Case Solved 44 HHMI LAB BOOK 46 N O T A B E N E 49 INSIDE HHMI Dollars and Sense ON THE COVER: Nancy H.