Effects of Climate Change and Heterogeneity of Local Climates on the Development of Malaria Parasite (Plasmodium Vivax) in Moscow Megacity Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moscow, Russia
Moscow, Russia INGKA Centres The bridge 370 STORES 38,6 MLN to millions of customers VISITORS ANNUALLY From families to fashionistas, there’s something for everyone meeting place where people connect, socialise, get inspired, at MEGA Belaya Dacha that connects people with inspirational experience new things, shop, eat and naturally feel attracted lifestyle experiences. Supported by IKEA, with more than to spend time. 370 stores, family entertainment and on-trend leisure and dining Our meeting places will meet people's needs & desires, build clusters — it’s no wonder millions of visitors keep coming back. trust and make a positive difference for local communities, Together with our partners and guests we are creating a great the planet and the many people. y w h e Mytischi o k v s la Khimki s o r a Y e oss e sh sko kov hel D RING RO c IR AD h ov Hwy TH S ziast ntu MOSCOW E Reutov The Kremlin Ryazansky Avenue Zheleznodorozhny Volgogradskiy Prospect Lyubertsy Kuzminki y Lyublino Kotelniki w H e o Malakhovka k s v a Dzerzhinsky h s r Zhukovskiy a Teply Stan V Catchment Areas People Distance Kashirskoe Hwy Lytkarino Novoryazanskoe Hwy ● Primary 1,600,000 < 20 km ● Secondary 1,600,000 20–35 km ● Tertiary 3,800,000 35–47 km Gorki Total area: <47 km: 7,000,000 Leninskiye Volodarskogo 55% 25 3 METRO 34 MIN CUSTOMERS BUS ROUTES STATIONS AVERAGE COME BY CAR NEAR BY COMMUTE TIME A region with Loyal customers MEGA Belaya Dacha is located at the heart of the very dynamic population development in strong potential the South-East of Moscow and attracts shoppers from all over Moscow and surrounding areas. -

MEGA Belaya Dacha Le N in G R Y a D W S H V K Olo O E K E O O Mytischi Lam H K Sk W S O Y Av E
MEGA Belaya Dacha Le n in g r y a d w s h V k olo o e k e o o Mytischi lam h k sk w s o y av e . sl o h r w a y Y M K Tver A Market overview D region Balashikha Dmitrov Krasnogorsk y Welcome v hw Sergiev-Posad hw uziasto oe y nt Klin Catchment Peoplesk Distance E Vladimir region izh or Reutov ov to MEGA N Mytischi Pushkin areas Schelkovo Belaya Dacha Moscow Zheleznodorozhny Primary 1,589,000 < 20 km Smolensk region Odintsovo N Naro-Fominsk o Podolsk v o ry a Klimovsk wy z Secondary 1,558,800 h 20–35 km a oe n k sk ins o Obninsk Kolomna M e y h hw w oe y Serpukhov Tertiary 3,787,300 35–47vsk km ALONG WITH LONDON’S WESTFIELD Kaluga region Kie AND ISTANBUL’S FORUM, MEGA BELAYA y y w Tula region h w h DACHA IS ONE OF EUROPE’S LARGEST e ko e Total area: 6,965,200 s o z h k RETAIL COMPLEXES. s lu Troitsk a v K a h s r a Domodedovo V It has more than 350 tenants and the centre Moscow has the highest density of retailers façade runs for four km. Major brands such of all Russian cities with tenants occupying as Auchan, Inditex brands, TopShop, H&M, 4.5 million square metres, according to fig- Uniqlo, T.G.I. Fridays, Debenhams, MAC, ures for 2013. Many world-famous retailers IKEA, OBI, MediaMarkt, Kinostar, Cosmic, have outlets here and the city is the first M.Video, Detsky Mir, Deti and Decathlon to show new trends. -

Revista Inclusiones Issn 0719-4706 Volumen 7 – Número Especial – Octubre/Diciembre 2020
CUERPO DIRECTIVO Mg. Amelia Herrera Lavanchy Universidad de La Serena, Chile Director Dr. Juan Guillermo Mansilla Sepúlveda Mg. Cecilia Jofré Muñoz Universidad Católica de Temuco, Chile Universidad San Sebastián, Chile Editor Mg. Mario Lagomarsino Montoya OBU - CHILE Universidad Adventista de Chile, Chile Editor Científico Dr. Claudio Llanos Reyes Dr. Luiz Alberto David Araujo Pontificia Universidad Católica de Valparaíso, Chile Pontificia Universidade Católica de Sao Paulo, Brasil Dr. Werner Mackenbach Editor Europa del Este Universidad de Potsdam, Alemania Dr. Aleksandar Ivanov Katrandzhiev Universidad de Costa Rica, Costa Rica Universidad Suroeste "Neofit Rilski", Bulgaria Mg. Rocío del Pilar Martínez Marín Cuerpo Asistente Universidad de Santander, Colombia Traductora: Inglés Ph. D. Natalia Milanesio Lic. Pauline Corthorn Escudero Universidad de Houston, Estados Unidos Editorial Cuadernos de Sofía, Chile Dra. Patricia Virginia Moggia Münchmeyer Portada Pontificia Universidad Católica de Valparaíso, Chile Lic. Graciela Pantigoso de Los Santos Editorial Cuadernos de Sofía, Chile Ph. D. Maritza Montero Universidad Central de Venezuela, Venezuela COMITÉ EDITORIAL Dra. Eleonora Pencheva Dra. Carolina Aroca Toloza Universidad Suroeste Neofit Rilski, Bulgaria Universidad de Chile, Chile Dra. Rosa María Regueiro Ferreira Dr. Jaime Bassa Mercado Universidad de La Coruña, España Universidad de Valparaíso, Chile Mg. David Ruete Zúñiga Dra. Heloísa Bellotto Universidad Nacional Andrés Bello, Chile Universidad de Sao Paulo, Brasil Dr. Andrés Saavedra Barahona Dra. Nidia Burgos Universidad San Clemente de Ojrid de Sofía, Bulgaria Universidad Nacional del Sur, Argentina Dr. Efraín Sánchez Cabra Mg. María Eugenia Campos Academia Colombiana de Historia, Colombia Universidad Nacional Autónoma de México, México Dra. Mirka Seitz Dr. Francisco José Francisco Carrera Universidad del Salvador, Argentina Universidad de Valladolid, España Ph. -

Moscow United Electric Grid Company” As of July 13, 2007 (Minutes No.46 As of July 17, 2007)
Approved by the Board of Directors decision of Open Joint-Stock Company “Moscow United Electric Grid Company” as of July 13, 2007 (Minutes No.46 as of July 17, 2007) Amendments No. 1 to the Charter Open Joint-Stock Company “Moscow United Electric Grid Company” To introduce the following amendments in the Charter of Open Joint-Stock Company “Moscow United Electric Grid Company”: To state the Company List of Branches (Appendix No. 1 to the Charter) as follows: List of Branches OJSC “Moscow United Electric Grid Company” No. Name Address 1. Central Electric Networks 115201, Moscow city, Kashirskoye highway, 18 2. Southern Electric Networks 115201, Moscow city, Kashirskoye highway, 18 3. Eastern Electric Networks 107140, Moscow city, Nizhnyaya Krasnoselskaya street, 6, bld. 1 4. Oktyabrskie Electric Netwowrks 127254, Moscow city, Rustaveli street, 2 5. Northern Electric Networks 141070, Moscow region, Korolev city, Gagarina street, 4 6. Noginsk Electric Networks 142400, Moscow region, Noginsk city, Radchenko street, 13 7. Podolsk Electric Networks 142117, Moscow region, Podolsk city, Kirova street, 65 8. Kolomna Electric Networks 140408, Moscow region, Kolomna city, Oktyabrskoy Revolyutsii street, 381а 9. Shatura Electric Networks 140700, Moscow region, Shatura city, Sportivnaya street, 12 10. Western Electric Networks 121170, Moscow city, 1812 Goda street, estate 15 11. Kashira Electric Networks 142900, Moscow region, Kashira city, Klubnaya street, 4 12. Mozhaisk Electric Networks 143200, Moscow region, Mozhaisk city, Mira street, 107 13. Dmitrov Electric Networks 141800, Moscow region, Dmitrov city, Kosmonavtov street, 46 14. Volokolamsk Electric Networks 143600, Moscow region, Volokolamsk city, Novosoldatskaya street, 58 15. Moskabelenergoremont (MKER) 115569, Moscow city, Shipilovskaya street, 13, bld. -
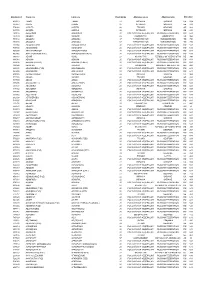
FÁK Állomáskódok
Állomáskód Orosz név Latin név Vasút kódja Államnév orosz Államnév latin Államkód 406513 1 МАЯ 1 MAIA 22 УКРАИНА UKRAINE UA 804 085827 ААКРЕ AAKRE 26 ЭСТОНИЯ ESTONIA EE 233 574066 ААПСТА AAPSTA 28 ГРУЗИЯ GEORGIA GE 268 085780 ААРДЛА AARDLA 26 ЭСТОНИЯ ESTONIA EE 233 269116 АБАБКОВО ABABKOVO 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 737139 АБАДАН ABADAN 29 УЗБЕКИСТАН UZBEKISTAN UZ 860 753112 АБАДАН-I ABADAN-I 67 ТУРКМЕНИСТАН TURKMENISTAN TM 795 753108 АБАДАН-II ABADAN-II 67 ТУРКМЕНИСТАН TURKMENISTAN TM 795 535004 АБАДЗЕХСКАЯ ABADZEHSKAIA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 795736 АБАЕВСКИЙ ABAEVSKII 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 864300 АБАГУР-ЛЕСНОЙ ABAGUR-LESNOI 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 865065 АБАГУРОВСКИЙ (РЗД) ABAGUROVSKII (RZD) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 699767 АБАИЛ ABAIL 27 КАЗАХСТАН REPUBLIC OF KAZAKHSTAN KZ 398 888004 АБАКАН ABAKAN 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 888108 АБАКАН (ПЕРЕВ.) ABAKAN (PEREV.) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 398904 АБАКЛИЯ ABAKLIIA 23 МОЛДАВИЯ MOLDOVA, REPUBLIC OF MD 498 889401 АБАКУМОВКА (РЗД) ABAKUMOVKA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 882309 АБАЛАКОВО ABALAKOVO 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 408006 АБАМЕЛИКОВО ABAMELIKOVO 22 УКРАИНА UKRAINE UA 804 571706 АБАША ABASHA 28 ГРУЗИЯ GEORGIA GE 268 887500 АБАЗА ABAZA 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 887406 АБАЗА (ЭКСП.) ABAZA (EKSP.) 20 РОССИЙСКАЯ ФЕДЕРАЦИЯ RUSSIAN FEDERATION RU 643 -

Russian Museums Visit More Than 80 Million Visitors, 1/3 of Who Are Visitors Under 18
Moscow 4 There are more than 3000 museums (and about 72 000 museum workers) in Russian Moscow region 92 Federation, not including school and company museums. Every year Russian museums visit more than 80 million visitors, 1/3 of who are visitors under 18 There are about 650 individual and institutional members in ICOM Russia. During two last St. Petersburg 117 years ICOM Russia membership was rapidly increasing more than 20% (or about 100 new members) a year Northwestern region 160 You will find the information aboutICOM Russia members in this book. All members (individual and institutional) are divided in two big groups – Museums which are institutional members of ICOM or are represented by individual members and Organizations. All the museums in this book are distributed by regional principle. Organizations are structured in profile groups Central region 192 Volga river region 224 Many thanks to all the museums who offered their help and assistance in the making of this collection South of Russia 258 Special thanks to Urals 270 Museum creation and consulting Culture heritage security in Russia with 3M(tm)Novec(tm)1230 Siberia and Far East 284 © ICOM Russia, 2012 Organizations 322 © K. Novokhatko, A. Gnedovsky, N. Kazantseva, O. Guzewska – compiling, translation, editing, 2012 [email protected] www.icom.org.ru © Leo Tolstoy museum-estate “Yasnaya Polyana”, design, 2012 Moscow MOSCOW A. N. SCRiAbiN MEMORiAl Capital of Russia. Major political, economic, cultural, scientific, religious, financial, educational, and transportation center of Russia and the continent MUSEUM Highlights: First reference to Moscow dates from 1147 when Moscow was already a pretty big town. -

Glass in Ancient and Medieval Eastern Europe As Evidence of International Contacts
Archeologia Polski 61 (2016), pp. 191-212 Archeologia Polski, LXI: 2016 PL ISSN 0003-8180 Ekaterina STOLYAROVA GLASS IN ANCIENT AND MEDIEVAL EASTERN EUROPE AS EVIDENCE OF INTERNATIONAL CONTACTS Abstract: This paper deals with glass artifacts as markers of interregional economic, religious and cultural links, trade routes, and social stratification. It is focused on finds from Eastern Europe from the Bronze Age to the 17th–18th centuries A.D. Keywords: glass beads, glass vessels, Eastern Europe, international links. Introduction Glass is one of the most ancient artificial materials possessing unique properties from which a variety of artifacts can be made. Among these are luxury artifacts and objects of applied art, tesserae for figured mosaics and stained glass, glass icons and ritual vessels, window-panes and tableware as well as small ornaments, i.e., arm rings, beads, fingerings, buttons and pendants. These artifacts were used in daily life, sold, donated, used to decorate clothes, interiors and architectural structures. They were symbols of their owner’s social and economic position. The value of glass as a historical source stems from its extensive application. Glass objects provide information on the formation and spread of glassmaking and on the place of glass in scientific concepts and the production of a given epoch. Chemical properties of glass and means of its production are of technological interest. Glass artifacts are important for the study of culture and daily life of a given epoch, e.g. the history of costume. Excavated glass objects are examined from the angle of their functions, peculiarities of their form and decoration, the spread and evolution of different type. -

MEGA Khimki Tver Region Market Overview Welcome
MEGA Khimki Tver region Market overview Welcome Dmitrov L e y n Sergiev-Posad Catchment areas People Distance i w y n h to MEGA Khimki Klin g w r a e h V Vladimir d o ol s e o k ko k o region la o s k m e v s Pushkin s Mytischi ko h o av e w r sl t Schelkovo y i o h . r a w m y Y Primary 398,200 < 17 km D Zheleznodorozhny M K A Smolensk Moscow D Balashikha region Podolsk Naro-Fominsk Secondary 1,424,200 17–40 km Krasnogorsk y Klimovsk v hw hw uziasto oe y nt RUSSIA’S FIRST IKEA WAS OPENED IN sk E Obninsk izh Kolomna or Reutov Tertiary 3,150,656 40–140 km ov KHIMKI IN 2000. MEGA KHIMKI SOON N Serpukhov FOLLOWED IN 2004 AND BECAME THE Kaluga region LARGEST RETAIL COMPLEX IN RUSSIA Tula region Total area: 4,973,000 AT THE TIME. Odintsovo N o v o ry y a hw z e a ko n s sk Min o e wy h h w oe y vsk Kie Despite several new retail centres opening their doors along the Leningradskoe Shosse, y y w w h MEGA Khimki remains one of the district’s h e oe o sk k most popular shopping destinations, largely s h Troitsk z Scherbinka v u a al due to its location, well-designed layout and K h s r retail mix. a V Domodedovo New tenants and constant improvements to the centre have significantly increased customer numbers. -

Industrial Framework of Russia. the 250 Largest Industrial Centers Of
INDUSTRIAL FRAMEWORK OF RUSSIA 250 LARGEST INDUSTRIAL CENTERS OF RUSSIA Metodology of the Ranking. Data collection INDUSTRIAL FRAMEWORK OF RUSSIA The ranking is based on the municipal statistics published by the Federal State Statistics Service on the official website1. Basic indicator is Shipment of The 250 Largest Industrial Centers of own production goods, works performed and services rendered related to mining and manufacturing in 2010. The revenue in electricity, gas and water Russia production and supply was taken into account only regarding major power plants which belong to major generation companies of the wholesale electricity market. Therefore, the financial results of urban utilities and other About the Ranking public services are not taken into account in the industrial ranking. The aim of the ranking is to observe the most significant industrial centers in Spatial analysis regarding the allocation of business (productive) assets of the Russia which play the major role in the national economy and create the leading Russian and multinational companies2 was performed. Integrated basis for national welfare. Spatial allocation, sectorial and corporate rankings and company reports was analyzed. That is why with the help of the structure of the 250 Largest Industrial Centers determine “growing points” ranking one could follow relationship between welfare of a city and activities and “depression areas” on the map of Russia. The ranking allows evaluation of large enterprises. Regarding financial results of basic enterprises some of the role of primary production sector at the local level, comparison of the statistical data was adjusted, for example in case an enterprise is related to a importance of large enterprises and medium business in the structure of city but it is located outside of the city border. -

Methods of Statistical Estimation of Circular Migration and Formal And
Statistical Journal of the IAOS 36 (2020) 535–547 535 DOI 10.3233/SJI-190604 IOS Press Methods of statistical estimation of circular migration and formal and informal employment in the Moscow agglomeration based on the integration of various data sources Polina Kriuchkovaa;b, Filipp Sleznovc;d;∗, Denis Fomchenkoc, Vladimir Laikamc and Igor Zakharchenkovc aThe Department of Economic Policy and Development, Moscow City Government, Moscow, Russia bHigher School of Economics, The National Research University, Moscow, Russia cThe Analytical Center of Moscow Government, Moscow, Russia dLomonosov Moscow State University, Moscow, Russia Abstract. Assessing circular migration, formal and informal employment and its spatiotemporal characteristics is a complex methodological and practical task for official statistics. A combination of various data sources, including official statistics, administrative data, and data from mobile operators, may provide new opportunities for obtaining circular migration, formal and informal employment estimates for the purposes of various levels of government, including the level of city management. The purpose of this paper is to demonstrate how the use of administrative data together with the mobile operators’ data can promptly improve the accuracy and informativeness of statistical indicators of the labor market including formal and informal employment, circular migration, etc. The population and employment in Moscow and in the Moscow agglomeration are the subjects of this paper. Authors combine several data sources such as the federal administrative data from the Pension Fund of the Russian Federation and the Federal Tax Authority, data from the Moscow city online public services, data from the mobile phone operators, as well as official statistical information provided by Russian Statistic Authority. -

The Holy Trinity in Russian Spirituality the Deep Stream of Russian Spirituality Continues to Inspire the Faithful in the USSR
they have pushed me away. Only prayer is left, but my mouth is dumb with grief. All mothers of the World, and Christians, I request your prayers, your defence and help; young Timofei, an orphan yet with a living mother, stretches out his child's hands to you. The Holy Trinity in Russian Spirituality The deep stream of Russian spirituality continues to inspire the faithful in the USSR. A channel into this stream was provided by a recent article (and particu larly by its footnotes) in the official Church publication, The Journal 0'£ the Moscow Patriarchate No. I, 1975 (pp. 63-80). Written by Archbishop Pitirim of V olokalamsk and entitled "The Church as the Realization of the Trinitarian Oikonomy", this article was originally presented as a report to the Uppsala conference, "Church Days - 74", held from 30 August-3 September, 1974. The extract printed below consists chiefly of footnotes, but these offer the ,.eader many riches. A live perception of the Triune God was natural to the Russian religious con sciousness from the very beginning. It is reflected not only in the rich liturgical inheritance of the Russian Orthodox Church, common to all Eastern Orthodoxy, but in the characteristic national features of the Russian ecclesiastical conscious ness. In ancient Lives of Saints, which was the favourite reading matter and prac tically the only means of spiritually educating the people, an important place is occupied by theological talks on the triune nature of God. Despite their ab stractness they penetrated deeply the consciousness of the Russian Christian and moulded him. -

Russia Missile Chronology
Russia Missile Chronology 2007-2000 NPO MASHINOSTROYENIYA | KBM | MAKEYEV DESIGN BUREAU | MITT | ZLATOUST MACHINE-BUILDING PLANT KHRUNICHEV | STRELA PRODUCTION ASSOCIATION | AAK PROGRESS | DMZ | NOVATOR | TsSKB-PROGRESS MKB RADUGA | ENERGOMASH | ISAYEV KB KHIMMASH | PLESETSK TEST SITE | SVOBODNYY COSMODROME 1999-1996 KRASNOYARSK MACHINE-BUILDING PLANT | MAKEYEV DESIGN BUREAU | MITT | AAK PROGRESS NOVATOR | SVOBODNYY COSMODROME Last update: March 2009 This annotated chronology is based on the data sources that follow each entry. Public sources often provide conflicting information on classified military programs. In some cases we are unable to resolve these discrepancies, in others we have deliberately refrained from doing so to highlight the potential influence of false or misleading information as it appeared over time. In many cases, we are unable to independently verify claims. Hence in reviewing this chronology, readers should take into account the credibility of the sources employed here. Inclusion in this chronology does not necessarily indicate that a particular development is of direct or indirect proliferation significance. Some entries provide international or domestic context for technological development and national policymaking. Moreover, some entries may refer to developments with positive consequences for nonproliferation 2007-2000: NPO MASHINOSTROYENIYA 28 August 2007 NPO MASHINOSTROYENIYA TO FORM CORPORATION NPO Mashinostroyeniya is set to form a vertically-integrated corporation, combining producers and designers of various supply and support elements. The new holding will absorb OAO Strela Production Association (PO Strela), OAO Permsky Zavod Mashinostroitel, OAO NPO Elektromekhaniki, OAO NII Elektromekhaniki, OAO Avangard, OAO Uralskiy NII Kompositsionnykh Materialov, and OAO Kontsern Granit-Elektron. While these entities have acted in coordination for some time, formation of the new corporation has yet to be finalized.