Mercury in Indian Coals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Town Survey Report, Dhanbad, Part XB, Series-4, Bihar
CENSUS OF INDIA 1981 PARTXB SERIES 4 BIHAR TOWN SURVEY REPORT DHANBAD Drafted by Rajendra Prasad Asstt. Director Edited by S.C. Saxena Deputy Director Supervised by V.K. Bhargava Deputy Director Directorate of Census Operations BIHAR FOREWORD Apart from the decennial enumeration of population, the Indian Census is ~teeped in the tradition of undertaking a variety of studies of topical interest. In fact, the publications brought out in connection with the earlier censuses contained veritable mines of informatfon!On racial, cultural, linguistic and a number of other aspects of life of the people of this country. With the advent of freedom, however, the scope and dimension of these special studies had to be re structured in a manner that would provide the basic feedbacks on the processes of development taking place in different spheres of life of the people especially under planned development. Thus, in connection with the 1961, Census, a massive programme wa~s launched inter-alia to conduct socio-economic survey of about 500 villages selected from different parts of the country. The main objective of this study was to know the way of life of the people living in Indian villages which accounted for 82 per cent of the total population as per the 1961 Census. There was, however, an imperative need to extand the area of the study to urban centres as well, to -provide a complete coverage of the people living in diverse sodo economic conditions. It was with this objective in view ancillary studies on towns were launched as part of the social studies programme in connection with the 1971 Census. -

Action Plan for Clusters of Dhanbad
ACTION PLAN FOR CLUSTERS OF DHANBAD _________________________________________________ JHARKHAND STATE POLLUTION CONTROL BOARD T A BUILDING, HEC COMPLEX, RANCHI 834004 1 (1) 1.0 INTRODUCTION 1.1 The territorial area of Nirsa block , industrial area of Govindpur block, municipal area of Dhanbad Sadar block, municipal area of Jharia block and industrial area of Sindri are included in the cluster of Dhanbad 1.2 Location: Satellite imagery is as given below. SATELLITE IMAGERY OF INDUSTRIAL CLUSTER OF DHANBAD SATELLITE IMAGERY OF THE AREA OF NIRSA BLOCK 2 SATELLITE IMAGERY OF DHANBAD SADAR BLOCK SATELLITE IMAGERY OF INDUSTRIAL AREA OF SINDRI 3 SATELLITE IMAGERY OF JHARIA BLOCK SATELLITE IMAGERY OF THE AREA OF GOBINDPUR 1.3 Digital map with demarcation of geographical boundaries and impact zones is as given below. 4 1.4 CEPI Score: Air- 64.50, Water- 59.00, Land -65.50, Total- 78.63 1.5 Total populations and sensitive receptors Hospital – Patliputra Medical College & Hospital, Dhanbad;Central Hospital, Jagjivan Nagar; Jamadoba Hospital; Fertilizer Hospital, Sindri Educational Institution – BIT Sindri, ISM Dhanbad, Patliputra Medical College, Dhanbad, RS More College, Govindpur, Sindri College, RSP College, Jharia, PK Roy College, SSLNT Womens College, BS College, Govt. Polytechnic, Dhanbad, Mining Institute, Dhanbad, Govt. Polytechnic, Bhaga; Mining institute, Bhaga; and around ten other colleges. Besides, there are around 500 schools. Court - The court of District and Session Judge is there at Dhanbad. TABLE-1 Details of population, its density, major surface water bodies, tourist spots, schools and the health care units 5 Name of block areas Sadar Jharia Govindpur Nirsa T Area (Sq. Km) 128.82 90.77 334.44 416.85 a Population (2001) 564468 475341 201876 376843 b Densityl 4382 5224 604 904 Majore Surface water Damodar, Damodar, Damodar, Damodar, body Barakar Barakar Barakar Barakar Tourist1 Spot NA NA NA Panchet & Maithan Dam Primary & Middle 155 119 144 215 School Healthcare unit 93 44 4 22 1.6 SourceE -- Govt. -

Dhanbad Division-A Historical Perspective
Dhanbad Division-A Historical Perspective Dhanbad is a premier coal loading division of Indian Railways. The first colliery line on this division was laid in 1894 and the Grand Chord section was opened in 1906. The division was initially created as a transportation sub-division of Asansol division in 1946. A full-fledged division became operational in 1963. Dhanbad division was initially one of the five divisions of the Eastern Railway but after the formation of ‘East Central Railway’ on 1stOctober, 2002,Dhanbad division became part of East Central Railway along with four other divisions i.e. Mughalsarai, Danapur, Sonepur and Samastipur with headquarter at Hajipur. Dhanbaddivision primarily loads coal.Most of the coal is carried to ‘Up’ country power houses located in Punjab, Haryana, Delhi and U.P. Coal is also loaded for industries (non-core) located in Northern India. Coal also moves for ‘Down’ country destinations viz. power houses located in West Bengal, and washed coal to steel plants located at Durgapur, IISCO Burnpur&Tisco. Other commodities loaded on the division are bauxite from Tori, Richughuta and Meralgram for Hindalco plant at Renukoot, lime stone/dolomite from Bhavnathpur for Bokaro steel plant and cement from ACC-Sindri and Lemos-Khalari. The territorial jurisdiction of the division covers Pradhankhunta(incl.)-Manpur(excl.), Gomoh-Barkakana-Garwa Road-Chopan, Chopan-Singrauli-Mahadiya, Karaila Road- Shaktinagar, Dhanbad-Sindri town, Dhanbad-Patherdih&Dhanbad-Chandrapura sections. It deals with traffic on two important routes namely G.C. (Grand Chord) section from Pradhankhunta to Manpur& the CIC (Central Industrial Coalfields) section from Gomoh- Barkakana-Garwa road. -

Pst On-14-01-2015 Sl Name Father Name Date of Address Categ Trade Commu Ipo/ Bd No
PST ON-14-01-2015 SL NAME FATHER NAME DATE OF ADDRESS CATEG TRADE COMMU IPO/ BD NO. REMARKS ROLL NO FOR NO. BIRTH ORY NITY WITH AMT ACCEPTED ACCEPTED AND DATE / CANDIDATES REJECTED 1 RANVEER SINGH YADAV DEV NATH YADAV 21-Aug-91 VILL-KUSTUR 4 GEN CT/DVR HINDU 85G 413056 ACCEPTED 1701100098 NO PO- DTD-12-12- KENDUADIH DIS- 14 RS -50 DHANBAD JKD- 828117 2 RAJNISH YADAV BINDHYACHAL YADAV 7-Apr-89 VILL-GODHAR GEN CT/DVR HINDU 85G 415271 ACCEPTED 1701100099 MORE PO- DTD-15-12- KUSUNDA PS- 14 RS -50 KENDUADIH DIS- DHANBAD JKD- 828117 3 SUNDARLAL SAHU SAHINDAR SAHU 7-Jul-89 VILL-KODHI PAAT GEN CT/DVR HINDU 74G-582635 ACCEPTED 1701100100 BARWATOLI PO- DTD 16 -12- GUNIYA PS- 14 RS-50 GHAGRA DIS- GUMLA JKD- 835208 4 MD SHAHJAD ALAM ABDUL MANNAN 8-Feb-90 VILL C/OMD GEN CT/DVR MUSLIM 85G-377534 ACCEPTED 1701100101 RABBANI KHAN DTD 15-12- LOKBIAR COLONY 14 RS-50 OPP LALPUR PO- +LAPUNG DIST- RANCHI JKD834001 5 JAYSHANKER PANDEY KUBER NATH PANDAY 1-Mar-88 GODHER BASTI GEN CT/DVR HINDU 85G 415268 ACCEPTED 1701100102 PO-KUSUNDA PS- DTD-15-12- KENDUADIH DIS- 14 RS -50 DHANBAD JKD- 828117 6 RAJESH SINGH YADAV GIRIJA SINGH YADAV 20-Jul-93 VILL-KUSTOR 4 GEN CT/DVR HINDU 85G 413057 ACCEPTED 1701100103 NO PO-KUSTOR DTD-12-12- PS-KENDUADIH 14 RS -50 DIS-DHANBAD JKD-828117 7 SATYAPAL SAHANI DINANATH SAHANI 3-Apr-88 VILL-KUSTOR NO GEN CT/DVR HINDU 85G 413059 ACCEPTED 1701100104 4 PO-KUSTOR PS- DTD-12-12- KENDUADIH DIS- 14 RS -50 DHANBAD JKD- 828117 8 AVADHESH YADAV SHIV KUMAR YADAV 1-May-89 VILL-GANSADIH GEN CT/DVR HINDU 85G 423950 ACCEPTED 1701100105 PO-KUSUNDA -

A Study in Industrial Health: Coal Miners in Eastern India, 1890S-1952
A Study in Industrial Health: Coal Miners in Eastern India, 1890s-1952 Dissertation Zur Erlangung des akademischen Grades Doctor philosophiae (Dr. phil.) eingereicht an der Philosophischen Fakultät der Georg August Universität, Göttingen Vorgelegt von Sandip Chatterjee March 2017 1. Gutachter/in: Prof. Dr. Ravi Ahuja, Georg-August-Universität Göttingen 2. Gutachter/in: Prof. Dr. Chitra Joshi, Humboldt-Universität zu Berlin, IGK Arbeit und Lebenslauf in globalgeschichtlicher Perspektive, Unter den Linden 6, 10099 Berlin 3. Gutachter/in: Prof. Dr. Dominic Sachsenmaier, Georg-August-Universität Göttingen Tag der mündlichen Prüfung……………………….. This dissertation is dedicated to my parents. Curriculum Vitae PERSONAL INFORMATION ________________________________________________ Name: SANDIP CHATTERJEE Date of Birth: 1 May, 1983 Marital Status: Single Gender: Male Citizenship: Indian CONTACT DETAILS ____________________________________________________________________________ Permanent Address: C/O- Debabrata Chatterjee P.O. South Garia, District: 24 Parganas (South) West Bengal-743613 India. Phone: +91 3218 260203 (Residence) +91 9830527441 (Mobile- India) e-mail: [email protected] Present Address: Same as above EDUCATIONAL QUALIFICATIONS _____________________________________________________________________________ Finished PhD on 12 June, 2017 from the Centre for Modern Indian Studies (CeMIS) , University of Göttingen, Germany. The title of my PhD thesis is “A Study in Industrial Health: Coal Miners in Eastern India, 1890s-1952.” I have worked under the supervision of Prof. Dr. Ravi Ahuja. M.A. (in History) from University of Calcutta in 2006 with 61 % of marks. B.A. (in History) from Presidency College, University of Calcutta in 2004 with 56 % of marks. Higher Secondary (in Arts) from West Bengal Council of Higher Secondary Education in 2001 with 71.1 % of marks. 1 Madhyamik from West Bengal Board of Secondary Education in 1999 with 79.5 % of marks. -
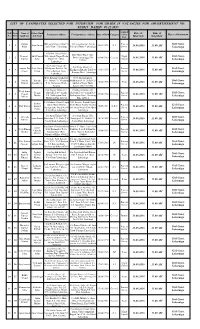
List of Candidates Selected for Interview .Pdf
LIST OF CANDIDATES SELECTED FOR INTERVIEW FOR GRADE IV VACANCIES FOR ADVERTISEMENT NO. 01/2015 DATED 05.11.2015 Applied Roll Form Name of Father/Husb Date of Time of Permanent Address Correspondence Address Date of Birth Category for the No. Sl.No Applicant and Name Place of Interview Post Interview Interview Indrani D/O jatru Oraon vill& P.S Vill juria Kathar Toli & Process Civil Court, 1 1 Jatru Oraon 30/03/1976 S.T 16.08.2016 11.00 AM Kujur juria Distt.- Lohardaga P.S juria Distt.- Lohardaga Server Lohardaga S/O Shiv Prasad Sahu Shiv Hotel Block More Pramod Shiv Prasad Vill- Shanti Nagar Kisko Night Civil Court, 2 2 Distt- Lohardaga PIN 05/06/1985 General 16.08.2016 11.00 AM Kumar Sahu More P.O + Distt- Guard 835302 Lohardaga Lohardaga S/o Nania Oraon vill- S/o Nania Oraon vill- Laxman Deo Late Nania Khakpatra Kumba Toli Process Civil Court, 3 3 Khakpatra Kumba Toli P.O 12/01/1976 S.T 16.08.2016 11.00 AM Oraon Oraon P.O – Rampur Distt- Server – Rampur Distt- Lohardaga Lohardaga Lohardaga D/O- Balram Upadhayay New Shashtri nagar Antima Balram Vill- Odnar P.S- Chainpur Madhukam,P.O- Hehal P.S- Civil Court, 4 4 12/06/1988 General Peon 16.08.2016 11.00 AM Tiwary Upadhayay P.O- Harinamar Distt- Sukhdeo Nagar Distt- Lohardaga Palamu Ranchi PIN – 834005 S/O Degan Mahto vill- S/O Degan Mahto vill- Bhadeshwar Degan Shivadih P.O – Sandh Shivadih P.O – Sandh P.S- Process Civil Court, 5 5 Prasad 09/06/1990 General Mahto P.S- Badkagaon Distt- Badkagaon Distt- Hazarbag Server 16.08.2016 11.00 AM Mehta Lohardaga Hazarbag Pin- 825311 Pin- 825311 S/O -
(ISM), Dhanbad 1 2
ANNUAL REPORT 2016 - 2017 INDIAN INSTITUTE OF TECHNOLOGY (INDIAN SCHOOL OF MINES) DHANBAD – 826 004 Annual Report 2016 - 2017 CONTENTS Page No. Foreword i Vision iii Mission iii Executive Summary v 1. About IIT (ISM), Dhanbad 1 2. Academic Activities 5 2.1 Undergraduate Programmes 6 2.2 Dual Degree Programmes 7 2.3 5-year Integrated Programmes 7 2.4 Postgraduate Programmes 8 2.5 Research Programmes 10 3. Research and Development 11 3.1 State-of-the-art Laboratories 11 3.2 Other Important Laboratories 11 3.3 Sponsored Research Projects 12 3.4 Ph.D. Degree Awarded 26 3.5 Research Publications 41 3.5.1 Publications in SCOPUS database during the calendar year 2016 41 3.5.2 Publications in Web of Science database during the calendar year 42 2016 3.5.3 List of Publications in SCOPUS database in the calendar year 43 2016 3.5.4 List of Publications in Web of Science database in the calendar 120 Year 2016 4. Centres of Advanced Learning 195 4.1 Central Research Facility (CRF) 195 4.2 Centre of Excellence in Safety, Occupational Health and Disaster 196 Management 4.2.1 Collaborative R&D projects with CIL and Australian Universities/ 196 Organizations under COESOHD 4.3 Australia-India Joint Centre of Excellence in Mining Technology 197 (CEMT) 4.3.1 Institution of Australian Partnership Coordinator (APC) position 197 4.3.2 Collaborative R&D Projects with CIL and Australian 197 Universities/ Organizations under CEMT 4.4 Oil and Gas Reservoir Characterization Simulation 197 Annual Report 2016 - 2017 5. -
Circle Place Containment Zone Buffer Zone Incident Commander Date
Containment Order Master Data Containment Zone Buffer Zone Date S.No Block/ Circle Place Incident Commander Block/DMC Enforcemen East West North South East West North South Withdrawal t Village - Dumdumi area of Parasi Lakhiyabad Ghoramur Fathehpur Smt. Vandana Bharti, Circle 1 Govindpur Tetuliya Dubhi Village East India Village Road Khudiya River Parasi Village Barwapurw 21.05.2020 04.06.2020 Block Panchyat Village ga Village Village Officer, Govindpur a Village Rodaband Sh.Ratan Kumar Singh, BDO, 2 Baliapur Saharpura Ward -55 Qr.No K-3/72 K1- Khatal Chowk K-3 /36, Gurudwara Road Rabindra Prasad More Sawalapur Rangamati Baradha 26.05.2020 09.06.2020 DMC h Baliapur Up to the House of Up to The hosue Of Birendra Kumar & Rishi Bhuli Hirak Baramudi Binodbihar Sh.Prashant Kumar Layak , 3 Dhanbad Baramuri , ward 21 Durga Mandir Bisunpur 27.05.2020 10.06.2020 DMC Mantosh Kumar Ranjeet Singh Raj Road Road i Chowk Circle Officer , Dhanbad Giridih Up to the House of Up to the House of Up to the Border of Kamta Sh. Vikas Kumar Trivedi, 4 Topchanchi Belmi Village, Ledatand Up to Raja Pahadi Kusumdiha District Pipratand 27.05.2020 10.06.2020 Block Sanichar Mahto Dumar Chand Mahto Purnatand Mouza Village Circle Officer, Topchanchi Border Sukudih Tola area of Gunghasa Up to the barren Land of Sukudih - Kherabera Up to Naya Prathmik Buiyachitr Chaita Sh. Vikas Kumar Trivedi, 5 Topchanchi Gomoh - Baghmara Road Barwadih Khesmi 28.05.2020 11.06.2020 Block Panchayat Abdul Rahman Road Vidhyalaya Aadiwasi Tola o Kherabeda Circle Officer, Topchanchi Govindpur- Sh. -

Government of Jharkhand
GOVERNMENT OF JHARKHAND Engineer-in-Chief-cum-Member Secretary Programme Management Unit Public Disclosure Authorized State Water & Sanitation Mission Drinking Water & Sanitation Department Ranchi, Jharkhand Doranda Market (Pani Tanki Campus), Doranda, Ranchi Phone: 0651-240920, 9431171291 Public Disclosure Authorized Environmental Assessment & Environmental Management Framework For the World Bank Assisted Water Supply Project in Selected Districts of Jharkhand Public Disclosure Authorized FINAL REPORT JULY-2013 Public Disclosure Authorized ENV Developmental Assistance Systems (India) Pvt Ltd B-5/131, Indira Nagar, Lucknow- 226018 Phone: 0522-2310576/77 E-mail: [email protected], Website: www.dasindia.org Study on Environment Assessment and Environment Management Framework for The World Bank assisted water supply project in the selected Districts of Jharkhand Environmental Assessment & Environmental Management Framework for The World Bank Assisted Water Supply Project in Selected Districts of Jharkhand TABLE OF CONTENTS SECTION SUBJECT PAGE NO EXECUTIVE SUMMARY A - G CHAPTER44TU - 1U44T 1 - 6 1 INTRODUCTION44T 44T 1 1.1 BACKGROUND 1 1.2 JHARKHAND STATE WATER AND SANITATION MISSION 1 1.2.1 MAIN OBJECTS 2 1.3 SCOPE OF STUDY 2 1.4 ENVIRONMENTAL ANALYSIS 2 1.5 THE STUDY AREA 3 1.6 APPROACH & METHODOLOGY 3 1.6.1 APPROACH 3 1.6.2 METHODOLOGY 3 1.6.2.1 DATA COLLECTION AND REVIEW 3 1.6.2.2 SAMPLE HABITATIONS FOR FIELD STUDY 4 1.6.2.3 SITE VISITS 5 1.6.3 METHODS FOR DATA COLLECTION 5 1.6.4 TOOLS FOR DATA COLLECTION 5 1.6.5 CONSULTATIONS 6 -

Dhanbad, Jharkhand Pen Cast Mining Land
International Research Journal of Environment Sciences _____________________________ ___ E-ISSN 2319–1414 Vol. 5(8), 65-71, August (2016) Int. Res. J. Environment Sci. Development Opportunities on Reclaimed Open Cast Mining Land: Dhanbad, Jharkhand Sanjeev Kumar 1 and Suman Sourav 2* 1Urban Planner, Society for Integrated Development of Habitat – Bhopal, India 2Urban Planner, Steaslit Systems Limited – Kolkata, India [email protected] Available online at: www.isca.in, www.isca.me Received 10 th May 2016, revised 24 th July 2016, accepted 19 th August 2016 Abstract Coal mining reclamation is a worldwide concern. As a result of the land reclamation development for opencast coal mine in India, the restoration and reclamation monitoring in Dhanbad has been noticed. Due to high ur banization rate in Dhanbad, per capita land availability is declining. There is a need to develop wasteland to cater the urban development demand of housing, agriculture, forestry, industries, transportation, recreational area etc. This paper presents a st udy where the land use aspects of reclamation are considered. The goal of the study is to transform the reclamation procedures into a physical process by integrating the use of land after reclamation and developing beneficial post operations. Study also pr oposes prospective development approach by assessing the current state of development of the reclaimed land in Jharia, Dhanbad coalfield region and to evaluate role of stakeholders for the proposed vision. In order to achieve this goal, a pilot survey was conducted to observe existing land use, and interviewed knowledgeable people and stakeholders in the case area. The study will able to: identify problems with the current state of urban development in Dhanbad, compare and contrast the needs and wants for r edevelopment of the area and determine the perspective development vision that how the land could be redeveloped to appease as many of the interest group as possible. -

Celebrations Kick Off As World Rings in 2017 Merkel: Islamist Terrorism
SUBSCRIPTION SUNDAY, JANUARY 1, 2017 RABI AL-THANI 3, 1438 AH www.kuwaittimes.net Kuwait Times offices will be closed on Sunday, January 1, 2017. Therefore, there will be no issue of the newspaper on Monday, January 2, 2017. Our next issue will hit the news- stands on Tuesday, January 3, 2017. Happy New Year! Celebrations kick off Min 09º Max 22º High Tide as world rings in 2017 13:12 & 23:25 Low Tide Revelers bid adieu to a year of conflicts 06:40 & 18:05 32 PAGES NO: 17098 150 FILS SYDNEY: Australia rang in the New Year with a spectacu- lar fireworks display in Sydney, sending rainbow-colored showers into the night sky and defying the global terror attacks that cast a pall over 2016. Around 1.5 million peo- ple packed Australia’s biggest city to watch as the mid- night fireworks erupted from Sydney Harbour Bridge, with the extravaganza beamed to television sets and phones across the world. As 2016 drew to a close, revelers around the world were biding a weary adieu to a year filled with political surprises, prolonged conflicts and deaths of legendary celebrities. Japan ushered in 2017 in style, with thousands pack- ing the streets of Tokyo and releasing balloons into the air in celebration of the new year. Sydney’s visual feast paid tribute to some of the international musical legends who died this year, including David Bowie and Prince, with purple rain pouring off the bridge in an early display and firework “stars” soaring high above the harbour. 2016 has seen repeated bloodshed, most recently a deadly truck attack at a Berlin Christmas market, a similar incident on Bastille Day in France that killed 86 people, and atrocities in Turkey and the Middle East. -

Annual Report (English).Pdf
Chapter Page No. I Highlights 2 II Organizational Structure and Functions of the Ministry of Steel 8 III The Indian Steel Sector : Development and Potential 11 IV Public Sector 18 V Private Sector 29 VI Research and Development 32 VII Energy, Environment Management and Climate Change 41 VIII Development of Information Technology 54 IX Safety 59 X Welfare of Weaker Sections of Society 65 XI Vigilance 68 XII Grievance Redressal Mechanism 75 XIII Implementation of Provisions of Persons with Disabilities Act. 1995 79 XIV Progressive use of Hindi 81 XV Empowerment of Women 87 XVI Promotion of Steel Usage 91 XVII Corporate Social Responsibility 94 XVIII Technical Institutes under the Ministry of Steel 103 XIX Implementation of the Right to Information Act, 2005 107 XX Development of North-Eastern Region 110 XXI International Cooperation 112 XXII Way Forward for Indian Steel Industry 113 Annexures 115-134 The production, financial and other related figures for 2016-17 are Provisional. CHAPTER-I HIGHLIGHTS 1.1 TRENDS AND DEVELOPMENTS IN STEEL SECTOR India is currently the world's 3rd largest producer of crude steel against its 8th position in 2003 and is expected to become the 2nd largest producer of crude steel in the world soon. India is the largest producer of direct reduced iron (DRI) or sponge iron in the world. The country is also the 3rd largest consumer of finished steel in the world preceded by China and the USA. Capacity for domestic crude steel production expanded from 90.87 million tonnes per annum (mtpa) in 2011-12 to 121.97 mtpa in 2015-16, a CAGR growth of 9% during this five year period.