Biochemical Pathways – Legends General Remarks for Part 1 and 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amphibolic Nature of Krebs Cycle
Amphibolic nature of Krebs Cycle How what we are is what we eat • In aerobic organisms, the citric acid cycle is an amphibolic pathway, one that serves in both catabolic and anabolic processes. • Since the citric acid does both synthesis (anabolic) and breakdown (catabolic) activities, it is called an amphibolic pathway • The citric acid cycle is amphibolic (i.e it is both anabolic and catabolic in its function). • It is said to be an AMPHIBOLIC pathway, because it functions in both degradative or catabolic and biosynthetic or anabolic reactions (amphi = both) A central metabolic pathway or amphibolic pathway is a set of reactions which permit the interconversion of several metabolites, and represents the end of the catabolism and the beginning of anabolism • The KREBS CYCLE or citric acid cycle is a series of reactions that degrades acetyl CoA to yield carbon dioxide, and energy, which is used to produce NADH, H+ and FADH. • The KREBS CYCLE connects the catabolic pathways that begin with the digestion and degradation of foods in stages 1 and 2 with the oxidation of substrates in stage 3 that generates most of the energy for ATP synthesis. • The citric acid cycle is the final common pathway in the oxidation of fuel molecules. In stage 3 of metabolism, citric acid is a final common catabolic intermediate in the form of acetylCoA. • This is why the citric acid cycle is called a central metabolic pathway. Anaplerosis and Cataplerosis Anaplerosis is a series of enzymatic reactions in which metabolic intermediates enter the citric acid cycle from the cytosol. Cataplerosis is the opposite, a process where intermediates leave the citric acid cycle and enter the cytosol. -

Exam #2 Review
Exam #2 Review Exam #2 will cover all the material that has been presented in class since Exam #1 and up through the metabolism introduction. This includes eukaryotic cell structure / function, transport, the closed system growth curve, enzymes and the introduction to metabolism. As always, it is best to begin by studying your notes and then after you feel your study is complete, take some time to look through this review. I. Eukaryotic cell structure / function A. There is a great deal of variance among eukaryotic cells - from protozoan cells to yeast cells to human cells. Fungi and protists (classically split into algae and protozoa) are eukaryotic representatives of the microbial world. B. Structure of the eukaryotic cell. 1. Cytoskeleton - provides structure and shape of cell, three components: a. Microtubules - largest element of cytoskeleton, composed of hollow cylinders of tubulin, form mitotic spindles, cilia and flagella and cell “highways”. b. Microfilaments - smallest element of cytoskeleton, composed of actin, involved in motion (pseudopod formation). c. Intermediate filaments - very stable structural element, play a supportive role, composed of proteins including keratin. Practice: Microfilaments a. are a component of the cytoskeleton. b. are long, twisted polymers of a protein called actin. c. form eukaryotic flagella. d. are made of tubulin. e. a and b f. c and d 2. Nucleus a. Bound by both an inner and outer membrane. The space between the two membranes is called the perinuclear space. The membrane has large nuclear pores through which proteins can pass (Why is this important?) b. Linear pieces of DNA are packaged by wrapping one and three quarters times around a histone octamer to form a core particle. -

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -
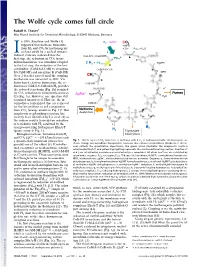
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

Untargetted Metabolomic Exploration of the Mycobacterium Tuberculosis Stress Response to Cinnamon Essential Oil
biomolecules Article Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil Elwira Sieniawska 1,* , Rafał Sawicki 2 , Joanna Golus 2 and Milen I. Georgiev 3,4 1 Chair and Department of Pharmacognosy, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland 2 Chair and Department of Biochemistry and Biotechnology, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland; [email protected] (R.S.); [email protected] (J.G.) 3 Group of Plant Cell Biotechnology and Metabolomics, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, 139 Ruski Blvd., 4000 Plovdiv, Bulgaria; [email protected] 4 Center of Plant Systems Biology and Biotechnology, 4000 Plovdiv, Bulgaria * Correspondence: [email protected] Received: 6 January 2020; Accepted: 24 February 2020; Published: 26 February 2020 Abstract: The antimycobacterial activity of cinnamaldehyde has already been proven for laboratory strains and for clinical isolates. What is more, cinnamaldehyde was shown to threaten the mycobacterial plasma membrane integrity and to activate the stress response system. Following promising applications of metabolomics in drug discovery and development we aimed to explore the mycobacteria response to cinnamaldehyde within cinnamon essential oil treatment by untargeted liquid chromatography–mass spectrometry. The use of predictive metabolite pathway analysis and description of produced lipids enabled the evaluation of the stress symptoms shown by bacteria. This study suggests that bacteria exposed to cinnamaldehyde could reorganize their outer membrane as a physical barrier against stress factors. They probably lowered cell wall permeability and inner membrane fluidity, and possibly redirected carbon flow to store energy in triacylglycerols. Being a reactive compound, cinnamaldehyde may also contribute to disturbances in bacteria redox homeostasis and detoxification mechanisms. -
Pyruvate Dehydrogenase
Aerobic Metabolism I: The Citric Acid Cycle Chapter 9 Overview n Live processes - series of oxidation-reduction reactions ¨ Ingestion of proteins, carbohydrates, lipids ¨ Provide basic building blocks for major molecules ¨ Produces energy n Aerobic metabolism I ¨ Citric Acid Cycle – series of reactions that release chemical energy stored in acetyl-CoA n Acetyl-CoA derived from pyruvate From McKee and McKee, Biochemistry, 5th Edition, © 2011 Oxford University Press Chapter 9: Overview §Citric acid cycle §Two-carbon fragments oxidized to form CO2 + §NAD /FAD reduced to NADH/FADH2 §Electron transport chain §Transfer of electrons from NADH/FADH2 to electron carriers §Terminal acceptor O2 §Oxidative phosphorylation §Energy released forms proton gradient §Drives ATP synthesis Figure 9.1 Overview of Aerobic Metabolism From McKee and McKee, Biochemistry, 5th Edition, © 2011 Oxford University Press Section 9.1: Oxidation-Reduction Reactions Figure 9.3 Reduction of Pyruvate by NADH §Redox reactions – electron transfer between an electron donor (reducing agent) & electron acceptor (oxidizing agent) §Many redox reactions have both an electron (e-) and a proton (H+) transferred §Conversion of pyruvate and NADH to lactate and NAD+ (shown above) is under anaerobic conditions From McKee and McKee, Biochemistry, 5th Edition, © 2011 Oxford University Press Section 9.1: Oxidation-Reduction Reactions §Half-reactions of redox reactions Cu loses e-, electron donor Cu+ ß à Cu2+ + e- Fe gains e-, electron acceptor Fe3+ + e- ß à Fe2+ Figure 9.4 An Electrochemical -

1 AMINO ACIDS Commonly, 21 L-Amino Acids Encoded by DNA Represent the Building Blocks of Animal, Plant, and Microbial Proteins
1 AMINO ACIDS Commonly, 21 L-amino acids encoded by DNA represent the building blocks of animal, plant, and microbial proteins. The basic amino acids encountered in proteins are called proteinogenic amino acids 1.1). Biosynthesis of some of these amino acids proceeds by ribosomal processes only in microorganisms and plants and the ability to synthesize them is lacking in animals, including human beings. These amino acids have to be obtained in the diet (or produced by hydrolysis of body proteins) since they are required for normal good health and are referred to as essential amino acids. The essential amino acids are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. The rest of encoded amino acids are referred to as non-essential amino acids (alanine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine, and tyrosine). Arginine and histidine are classified as essential, sometimes as semi-essential amino acids, as their amount synthesized in the body is not sufficient for normal growth of children. Although it is itself non-essential, cysteine (classified as conditionally essential amino acid) can partly replace methionine, which is an essential amino acid. Similarly, tyrosine can partly replace phenylalanine. 1.1 The glutamic acid group 1.1.1 Glutamic acid and glutamine Free ammonium ions are toxic to living cells and are rapidly incorporated into organic compounds. One of such transformations is the reaction of ammonia with 2-oxoglutaric acid from the citric acid cycle to produce L-glutamic acid. This reaction is known as reductive amination. Glutamic acid is accordingly the amino acid generated first as both constituent of proteins and a biosynthetic precursor. -

Sample Thesis Title with a Concise And
DESIGN, SYNTHESIS AND STUDIES OF GUANIDINIUM-BASED INHIBITORS FOR ISOPRENOID BIOSYNTHETIC PATHWAY ENZYMES by Walid Abdelmagid MChem., The University of Liverpool, 2011 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Doctor of Philosophy in THE FACULTY OF GRADUATE AND POSTDOCTORAL STUDIES (Chemistry) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) October 2019 © Walid Abdelmagid, 2019 i The following individuals certify that they have read, and recommend to the Faculty of Graduate and Postdoctoral Studies for acceptance, the dissertation entitled: DESIGN, SYNTHESIS AND STUDIES OF GUANIDINIUM-BASED INHIBITORS FOR ISOPRENOID BIOSYNTHETIC PATHWAY ENZYMES submitted by Walid Abdelmagid in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemistry Examining Committee: Martin Tanner Supervisor Stephen Withers Supervisory Committee Member Glenn Sammis Supervisory Committee Member Kathryn Ryan University Examiner David Chen University Examiner Additional Supervisory Committee Members: Supervisory Committee Member Supervisory Committee Member ii Abstract In this thesis an inhibition strategy was developed to target enzymes that utilize allylic diphosphates. Positively-charged inhibitors that mimic the transition states/intermediates formed with these enzymes were synthesized. In chapter two, inhibitor 2 containing a guanidinium moiety appended to a phosphonylphosphinate was designed to mimic the transition state for the dissociation of dimethylallyl diphosphate into an allylic carbocation-pyrophosphate ion-pair. To test for the effectiveness of incorporating a guanidinium functionality into inhibitors of human farnesyl diphosphate synthase, inhibitors 3 and 4 were also prepared. Inhibitor 3 has a positive charge localized onto one atom, and inhibitor 4 is isosteric to inhibitor 2, but lacks positive charge. The inhibitors displayed IC50 values that were significantly higher than the substrate Km value, indicating that the positive charge did not result in tight binding to the enzyme. -

Xuejun Yu Dissertation Final
UNIVERSITY OF CALIFORNIA RIVERSIDE Conversion of Carbon Dioxide to Formate by a Formate Dehydrogenase from Cupriavidus necator A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Bioengineering by Xuejun Yu September 2018 Dissertation Committee: Dr. Ashok Mulchandani, Co-Chairperson Dr. Xin Ge, Co-Chairperson Dr. Russ Hille Copyright by Xuejun Yu 2018 The Dissertation of Xuejun Yu is approved: Committee Co-Chairperson Committee Co-Chairperson University of California, Riverside ACKNOWLEDGEMENTS I would like to express sincere appreciation to my advisor, Professor Ashok Mulchandani, for accepting me to be his student when I was suffering. Thank you very much for your delicate guidance, support and encouragement during the past years. You not only guide me on my PhD study, but also help me to be mature on my personality. From bottom of my heart, I feel very lucky to have you as professor. Also, many thanks go to my other committee members. Professor Xin Ge acted as my co-advisor and provided many valuable comments on my molecular cloning work. Professor Russ Hille has also provided insights, encouragements and advices as a member of my committee members. I have learned so many important insights from our meetings and discussions and thank you for bringing me into the molybdenum/tungsten enzyme conference. I am also thankful to the past and current group members, colleagues and friends - Dimitri Niks, Pankaj Ramnani, Feng Tan, Rabeay Hassan, Trupti Terse, Thien-Toan Tran, Claudia Chaves, Jia-wei Tay, Hui Wang, Pham Tung, Hilda Chan, Yingning Gao and Tynan Young. -

Catabolism of the Carbon Skeletons of Amino Acids
Bio. 2. ASPU. Lectu.3. Prof. Dr. F. ALQuobaili Catabolism of the Carbon Skeletons of Amino Acids • Biomedical Importance ‐ The metabolic diseases or "inborn errors of metabolism" associated with conversion of the carbon skeletons of the common L ‐‐amino acids to amphibolic intermediates can result in irreversible brain damage and .(ﺍﻟﻭﻓﺎﺓ) early mortality ‐ Prenatal or early postnatal detection and timely initiation of treatment thus are essential. Almost all states conduct screening tests for up to as many as 30 metabolic diseases. ‐ The best screening tests use tandem mass spectrometry to detect, in a few drops of neonate blood, catabolites suggestive of a metabolic defect. ‐ Treatment consists primarily of feeding diets low in the amino acids whose catabolism is impaired. • Transamination Typically Initiates Amino Acid Catabolism Removal of ‐amino nitrogen by transamination is the first catabolic reaction of amino acids except for proline, hydroxyproline, threonine, and lysine. The hydrocarbon skeleton that remains is then degraded to amphibolic intermediates. • Asparagine, Aspartate, Glutamine, and Glutamate All four carbons of asparagine and aspartate form oxaloacetate. Analogous reactions convert glutamine and glutamate to ‐ ketoglutarate. No metabolic defects are associated with the catabolism of these four amino acids. • Proline The catabolism of proline takes place in mitochodria. Since proline does not participate in transamination, the nitrogen of this imino acid is retained throughout its oxidation to 1‐ pyrolline‐5‐carboxylate, ring opening to glutamate‐‐ semialdehyde, and oxidation to glutamate, and is only removed during transamination of glutamate to ‐ketoglutarate. There are two metabolic disorders of proline catabolism. Both types are inherited as autosomal recessive traits, and are consistent with a normal adult life. -

WO 2008/073367 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 19 June 2008 (19.06.2008) WO 2008/073367 Al (51) International Patent Classification: Quinn, Qun [US/US], 544 Revere Road, West Chester, C12N 1/16 (2006 01) C07C 403/24 (2006 01) Pennsylvania 19381 (US) C12N 1/15 (2006 01) (74) Agent: FELTHAM, S., NeU, E I du Pont de Nemours and (21) International Application Number: Company, Legal Patent Records Center, 4417 Lancaster PCT/US2007/025222 Pike, Wilmington, Delaware 19805 (US) (22) International Filing Date: (81) Designated States (unless otherwise indicated for every 10 December 2007 (10 12 2007) kind of national protection available): AE, AG, AL, AM, (25) Filing Language: English AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY,BZ, CA, CH, CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, (26) Publication Language: English ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, (30) Priority Data: IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, 60/869,576 12 December 2006 (12 12 2006) US LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, 60/869,574 12 December 2006 (12 12 2006) US MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, 60/869,591 12 December 2006 (12 12 2006) US PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, 60/869,582 12 December 2006 (12 12 2006) US TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, 60/869,580 12 December 2006 (12 12 2006) US ZM, ZW (71) Applicant (for all designated States except US): E.