Corrosion Fatigue of Austenitic Stainless Steels for Nuclear Power Engineering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corrosion Fatigue: Type 304 Stainless Steel in Acid-Chloride and Implant Metals in Biological Fluid
76-9939 BOWERS, Davtd Francis, 1937- CORROSION FATIGUE: TYPE 304 STAINLESS STEEL IN ACID-CHLORIDE AND IMPLANT METALS IN BIOLOGICAL FLUID. The Ohio State University, Ph.D., 1975 Engineering, metallurgy Xerox University Microfilms, Ann Arbor, Michigan 48ioe CORROSION FATIGUE: TYPE 304 STAINLESS STEEL IN ACID-CHLORIDE AND IMPLANT METALS IN BIOLOGICAL FLUID Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University by David Francis Bowers, B.S., M.S. Metallurgical Engineering The Ohio State University 1975 Approved by (dviser Department of Metallurgical Engineering Reading Committee: Dr. Mars G. Fontana Dr. Frank Beck Dr. John P. Hirth To My Wife and Daughter TABLE OF CONTENTS Page ACKNOWLEDGEMENTS.......................................... iv VITA........................................................ vi LIST OF TABLES............................................. viii LIST OF FIGURES... ...................... x INTRODUCTION.................. .. ......................... 1 I. LITERATURE SURVEY..................... 4 1.1 Concepts and Theory of Fatigue Crack Growth.. 4 1.2 Fracture Mechanics Approach to Crack Growth in Engineering Structures..................... 18 1.3 Effect of Microstructure on Fatigue Cracking Growth................................ 24 1.4 Mechanical Variables in Corrosion Fatigue.... 41 1.5 Environmental Interaction with Fatigue Crack Growth.................................... 59 II. EXPERIMENTAL DETAILS............................... -

Top Performance When the Going Gets Tough
Top performance when the going gets tough The Alfa Laval DuroShell plate-and-shell heat exchanger Sub-headline coming soon DuroShell – plate-and-shell made tougher Alfa Laval DuroShell is a specially engineered plate-and-shell heat exchanger ideal for demanding duties and corrosive media. Able to withstand fatigue even at high temperatures and pressures, it outperforms not only traditional heat exchangers, but also other plate-and-shells. As flexible as it is strong DuroShell creates new possibilities through its com- pactness, efficiency and exceptional resistance to fatigue. Able to work with liquids, gases and two- phase mixtures, it stands out among heat exchangers in its duty range. DuroShell handles pressures up to 100 barg (1450 psig) in compliance with PED and ASME, and temperatures as high as 450 °C (842 °F). Built for your application DuroShell is fully welded and gasket-free, with internal features that make it still more robust. Plates are avail- able in 316L stainless steel, while the pressure vessel itself can be built in 316L stainless steel or carbon steel. Three different sizes are possible, with heat transfer surfaces ranging 2–235 m2 (21.5–2530 ft 2) in area. DuroShell RollerCoaster Robust and efficient performance. DuroShell PowerPack Optimized flow distribution and fatigue resistance. Learn more at www.alfalaval.com/duroshell Plate-and-shell benefits made better • More uptime and longer life due to • Installation savings through even greater fatigue resistance more compact, lightweight design • Higher operating pressures thanks • Greater reliability as a result of to robust, patented construction closed, fully welded construction • Operational gains created by 15–20 % higher thermal efficiency How it works Revolutionary technology Optimized flow DuroShell is a plate-and-shell heat exchanger, but one DuroShell operates with one media on the plate side with a unique internal design. -

Corrosion-Fatigue Evaluation of Uncoated Weathering Steel Bridges
applied sciences Article Corrosion-Fatigue Evaluation of Uncoated Weathering Steel Bridges Yu Zhang, Kaifeng Zheng, Junlin Heng * and Jin Zhu Department of Bridge Engineering, School of Civil Engineering, Southwest Jiaotong University, Chengdu 610031, China * Correspondence: [email protected]; Tel.: +86-182-8017-6652 Received: 19 July 2019; Accepted: 19 August 2019; Published: 22 August 2019 Featured Application: The new findings highlight the enhancement in corrosion-fatigue performance of steel bridges by using the uncoated weathering steel. Besides, an innovative approach has been established to simulate the corrosion-fatigue process in uncoated weathering steel bridges, providing deep insights into the service life of the bridges. The approaches suggested in the article can be used (not limited to) as a reference for the research and design of uncoated weathering steel bridges. Abstract: Uncoated weathering steel (UWS) bridges have been extensively used to reduce the lifecycle cost since they are maintenance-free and eco-friendly. However, the fatigue issue becomes significant in UWS bridges due to the intended corrosion process utilized to form the corrodent-proof rust layer instead of the coating process. In this paper, an innovative model is proposed to simulate the corrosion-fatigue (C-F) process in UWS bridges. Generally, the C-F process could be considered as two relatively independent stages in a time series, including the pitting process of flaw-initiation and the fatigue crack propagation of the critical pitting flaw. In the proposed C-F model, Faraday’s law has been employed at the critical flaw-initiation stage to describe the pitting process, in which the pitting current is applied to reflect the pitting rate in different corrosive environments. -

Fatigue Overview Introduction to Fatigue Analysis
Fatigue overview Introduction to fatigue analysis • Fatigue is the failure of a component after several repetitive load cycles. • As a one-time occurrence, the load is not dangerous in itself. Over time the alternating load is able to break the structure anyway. • It is estimated that between 50 and 90 % of product failures is caused by fatigue, and based on this fact, fatigue evaluation should be a part of all product development. What is fatigue? In materials science, fatigue is the progressive and localized structural damage that occurs when a material is subjected to cyclic loading (material is stressed repeatedly). Clients tous différents Routes de qualités variables Contraintes Fatigue Design in Automotive Industry Conception fiable PSA (Peugeot Citroën) Résistances 3s 3s Dispersion matériau Dispersion de production Fatigue • Fracture mechanics can be divided into three stages: 1. Crack nucleation 2. Crack-growth 3. Ultimate ductile failure Introduction to fatigue analysis • Fatigue is the failure of a component after several repetitive load cycles. • As a one-time occurrence, the load is not dangerous in itself. Over time the alternating load is able to break the structure anyway. • It is estimated that between 50 and 90 % of product failures is caused by fatigue, and based on this fact, fatigue evaluation should be a part of all product development. Historical background • In comparison to the classical stress analysis, fatigue theory is a relative new phenomenon. The need to understand fatigue arose after the industrial revolution introduced steel structures. Three areas were particularly involved in early failures: Railway trains, Mining equipment and Bridges. Historical background • 1837: Wilhelm Albert publishes the first article on fatigue. -
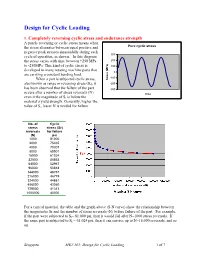
Design for Cyclic Loading, Soderberg, Goodman and Modified Goodman's Equation
Design for Cyclic Loading 1. Completely reversing cyclic stress and endurance strength A purely reversing or cyclic stress means when the stress alternates between equal positive and Pure cyclic stress negative peak stresses sinusoidally during each 300 cycle of operation, as shown. In this diagram the stress varies with time between +250 MPa 200 to -250MPa. This kind of cyclic stress is 100 developed in many rotating machine parts that 0 are carrying a constant bending load. -100 When a part is subjected cyclic stress, Stress (MPa) also known as range or reversing stress (Sr), it -200 has been observed that the failure of the part -300 occurs after a number of stress reversals (N) time even it the magnitude of Sr is below the material’s yield strength. Generally, higher the value of Sr, lesser N is needed for failure. No. of Cyclic stress stress (Sr) reversals for failure (N) psi 1000 81000 2000 75465 4000 70307 8000 65501 16000 61024 32000 56853 64000 52967 96000 50818 144000 48757 216000 46779 324000 44881 486000 43060 729000 41313 1000000 40000 For a typical material, the table and the graph above (S-N curve) show the relationship between the magnitudes Sr and the number of stress reversals (N) before failure of the part. For example, if the part were subjected to Sr= 81,000 psi, then it would fail after N=1000 stress reversals. If the same part is subjected to Sr = 61,024 psi, then it can survive up to N=16,000 reversals, and so on. Sengupta MET 301: Design for Cyclic Loading 1 of 7 It has been observed that for most of engineering materials, the rate of reduction of Sr becomes negligible near the vicinity of N = 106 and the slope of the S-N curve becomes more or less horizontal. -

The Environmental Effect on Corrosion Fatigue Behavior of Austenitic Stainless Steels
The Environmental Effect on Corrosion Fatigue Behavior of Austenitic Stainless Steels by Lun Yu B.S. Nuclear Engineering (2013) Shanghai Jiao Tong University SUBMITTED TO THE DEPARTMENT OF NUCLEAR SCIENCE AND ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN NUCLEAR SCIENCE AND ENGINEERING AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY September, 2017 © 2017 Massachusetts Institute of Technology. All rights reserved. Signature of Author: _____________________________________________________________ Lun Yu Department of Nuclear Science and Engineering August 25, 2017 Certified by: __________________________________________________________________ Ronald G. Ballinger Professor of Nuclear Science and Engineering, Materials Science and Engineering Thesis Supervisor Certified by: ___________________________________________________________________ Michael P. Short Assistant Professor of Nuclear Science and Engineering Thesis Reader Accepted by: ___________________________________________________________________ Ju Li Battelle Energy Alliance Professor of Nuclear Science and Engineering, Professor of Materials Science and Engineering Chair, Department Committee on Graduate Students This page was left blank intentionally The Environmental Effect on Corrosion Fatigue Behavior of Austenitic Stainless Steels by Lun Yu Submitted to the Department of Nuclear Science and Engineering on September 1, 2017 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Nuclear Science and -

Very High Cycle Fatigue of Engineering Materials
Faculty of Technology and Science Materials Engineering Vitaliy Kazymyrovych Very high cycle fatigue of engineering materials (A literature review) Karlstad University Studies 2009:22 Vitaliy Kazymyrovych Very high cycle fatigue of engineering materials (A literature review) Karlstad University Studies 2009:22 Vitaliy Kazymyrovych. Very high cycle fatigue of engineering materials - A literature review Research Report Karlstad University Studies 2009:22 ISSN 1403-8099 ISBN 978-91-7063-246-4 © The Author Distribution: Faculty of Technology and Science Materials Engineering SE-651 88 Karlstad +46 54 700 10 00 www.kau.se Printed at: Universitetstryckeriet, Karlstad 2009 Very high cycle fatigue of engineering materials (A literature review) V.Kazymyrovych* Department of Materials Engineering, Karlstad University SE-651 88, Sweden * Email address: [email protected] Abstract This paper examines the development and present status of the Very High Cycle Fatigue (VHCF) phenomenon in engineering materials. The concept of ultrasonic fatigue testing is described in light of its historical appearance covering the main principles and equipment variations. The VHCF behaviour of the most important materials used for very long life applications is presented, with particular attention paid to steels. In section 3 the VHCF properties of titanium-, nickel-, aluminium- and magnesium alloys are described. Furthermore, the typical fatigue behaviour and mechanisms of pure metals are presented. Section 4 examines the VHCF properties of various types of steels e.g. low carbon steel, spring steel, stainless steel, bearing steel as well as tool steel. In addition to this, the main material defects that initiate VHCF failure are examined in this study. Furthermore, the different stages characteristic for fatigue crack development in VHCF are described in section 5 in terms of relative importance and sequence. -

10Th International Congress on Marine Corrosion and Fouling, University of Melbourne, February 1999
10th International Congress on Marine Corrosion and Fouling, University of Melbourne, February 1999 Additional Papers John A Lewis (Editor) Maritime Platforms Division Aeronautical and Maritime Research Laboratory DSTO-GD-0287 ABSTRACT This volume contains nineteen papers from the 10th International Congress on Marine Corrosion and Fouling, held at the University of Melbourne in Melbourne, Australia, in February 1999. The scope of the congress was to enhance scientific understanding of the processes and prevention of chemical and biological degradation of materials in the sea. Papers in this volume range across the themes of marine biofilms and bioadhesion, macrofouling processes and effects, methods for prevention of marine fouling, biocides in the marine environment, biodeterioration of wood in the sea, and marine corrosion. RELEASE LIMITATION Approved for public release Published by DSTO Aeronautical and Maritime Research Laboratory 506 Lorimer St Fishermans Bend, Victoria 3207 Australia Telephone: (03) 9626 7000 Fax: (03) 9626 7999 © Commonwealth of Australia 2001 AR-011-880 May 2001 APPROVED FOR PUBLIC RELEASE 10th International Congress on Marine Corrosion and Fouling, University of Melbourne, February 1999 Additional Papers Executive Summary The fouling and corrosion of vessels and structures immersed in the sea continues to pose significant economic and operational costs to the owner. Fouling growth can interfere with the operation of submerged equipment, impose increased loading stresses and accelerate corrosion on marine structures, and adversely affect the performance of ships by increasing hydrodynamic drag, which necessitates the use of more power and fuel to move the ship through the water. Similarly, marine corrosion and biodegradation of materials can compromise the operation and structural integrity of vessels, structures and other immersed equipment. -
Four Types of Heat Exchanger Failures
Four Types of Heat Exchanger Failures . mechanical, chemically induced corrosion, combination of mechanical and chemically induced corrosion, and scale, mud, and algae fouling By MARVIN P. SCHWARTZ, Chief Product Engineer, ITT Bell & Gossett a unit of Fluid Handling Div., International Telephone & Telegraph Corp , Skokie IL Hcat exchangers usually provide a long service life with Maximum recommended velocity in the tubes and little or no maintenance because they do not contain any entrance nozzle is a function of many variables, including moving parts. However, there are four types of heat tube material, fluid handled, and temperature. Materials exchanger failures that can occur, and can usually be such as steel, stainless steel, and copper-nickel withstand prevented: mechanical, chemically induced corrosion, higher tube velocities than copper. Copper is normally combination of mechanical and chemically induced limited to 7.5 fps; the other materials can handle 10 or 11 corrosion, and scale, mud. and algae fouling. fps. If water is flowing through copper tubing, the velocity This article provides the plant engineer with a detailed should be less than 7.5 fps when it contains suspended look at the problems that can develop and describes the solids or is softened. corrective actions that should be taken to prevent them. Erosion problems on the outside of tubes usually result Mechanical-These failures can take seven different from impingement of wet, high-velocity gases, such as forms: metal erosion, steam or water hammer, vibration, steam. Wet gas impingement is controlled by oversizing thermal fatigue, freeze-up, thermal expansion. and loss of inlet nozzles, or by placing impingement baffles in the cooling water. -

Creep-Fatigue Failure Diagnosis
Review Creep-Fatigue Failure Diagnosis Stuart Holdsworth Received: 22 October 2015 ; Accepted: 6 November 2015 ; Published: 16 November 2015 Academic Editor: Robert Lancaster EMPA: Swiss Federal Laboratories for Materials Science and Technology Überlandstrasse 129, Dübendorf CH-8600, Switzerland; [email protected]; Tel.: +41-58-765-47-32 Abstract: Failure diagnosis invariably involves consideration of both associated material condition and the results of a mechanical analysis of prior operating history. This Review focuses on these aspects with particular reference to creep-fatigue failure diagnosis. Creep-fatigue cracking can be due to a spectrum of loading conditions ranging from pure cyclic to mainly steady loading with infrequent off-load transients. These require a range of mechanical analysis approaches, a number of which are reviewed. The microstructural information revealing material condition can vary with alloy class. In practice, the detail of the consequent cracking mechanism(s) can be camouflaged by oxidation at high temperatures, although the presence of oxide on fracture surfaces can be used to date events leading to failure. Routine laboratory specimen post-test examination is strongly recommended to characterise the detail of deformation and damage accumulation under known and well-controlled loading conditions to improve the effectiveness and efficiency of failure diagnosis. Keywords: failure diagnosis; creep-fatigue; material condition; mechanical analysis 1. Introduction The diagnosis of failures invariably involves consideration of both the associated material condition and the results of a mechanical analysis of prior operating history. Material condition refers not only to a knowledge of the chemical composition and mechanical properties relative to those originally specified for the failed component, but also the appearance and extent of microstructural and physical damage responsible for failure. -

Creep, Fatigue and Creep-Fatigue Interactions in Modified 9% Cr - 1% Mo (P91) Steels" (2013)
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 5-2013 Creep, Fatigue and Creep-Fatigue Interactions in Modified 9% rC - 1% Mo (P91) Steels Valliappa Kalyanasundaram University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/etd Part of the Mechanics of Materials Commons, Structural Engineering Commons, and the Structural Materials Commons Recommended Citation Kalyanasundaram, Valliappa, "Creep, Fatigue and Creep-Fatigue Interactions in Modified 9% Cr - 1% Mo (P91) Steels" (2013). Theses and Dissertations. 692. http://scholarworks.uark.edu/etd/692 This Dissertation is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. CREEP, FATIGUE AND CREEP-FATIGUE INTERACTIONS IN MODIFIED 9% Cr – 1% Mo (P91) STEELS CREEP, FATIGUE AND CREEP-FATIGUE INTERACTIONS IN MODIFIED 9% Cr – 1% Mo (P91) STEELS A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Mechanical Engineering By Valliappa Kalyanasundaram Madurai Kamaraj University Bachelor of Engineering in Mechanical Engineering, 2004 University of Arkansas Master of Science in Mechanical Engineering, 2008 May 2013 University of Arkansas ABSTRACT Grade P91 steel, from the class of advanced high-chrome ferritic steels, is one of the preferred materials for many elevated temperature structural components. Creep-fatigue (C-F) interactions, along with oxidation, can accelerate the kinetics of damage accumulation and consequently reduce such components’ life. Hence, reliable C-F test data is required for meticulous consideration of C-F interactions and oxidation, which in turn is vital for sound design practices. -

Analysis of Fatigue and Wear Behaviour in Ultrafine Grained Connecting Rods
metals Article Analysis of Fatigue and Wear Behaviour in Ultrafine Grained Connecting Rods Rodrigo Luri, Carmelo J. Luis, Javier León, Juan P. Fuertes, Daniel Salcedo and Ignacio Puertas * Mechanical, Energetics and Materials Engineering Department, Public University of Navarre, Campus Arrosadía s/n, Pamplona 31006, Spain; [email protected] (R.L.); [email protected] (C.J.L.); [email protected] (J.L.); [email protected] (J.P.F.); [email protected] (D.S.) * Correspondence: [email protected]; Tel.: +34-948-169-305 Received: 3 July 2017; Accepted: 20 July 2017; Published: 29 July 2017 Abstract: Over the last few years there has been an increasing interest in the study and development of processes that make it possible to obtain ultra-fine grained materials. Although there exists a large number of published works related to the improvement of the mechanical properties in these materials, there are only a few studies that analyse their in-service behaviour (fatigue and wear). In order to bridge the gap, in this present work, the fatigue and wear results obtained for connecting rods manufactured by using two different aluminium alloys (AA5754 and AA5083) previously deformed by severe plastic deformation (SPD), using Equal Channel Angular Pressing (ECAP), in order to obtain the ultrafine grain size in the processed materials are shown. For both aluminium alloys, two initial states were studied: annealed and ECAPed. The connecting rods were manufactured from the previously processed materials by using isothermal forging. Fatigue and wear experiments were carried out in order to characterize the in-service behaviour of the components.