CNS Distribution of Members of the Two-Pore-Domain (KCNK) Potassium Channel Family
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Sensors of the Pain Pathway
Nociceptors: the sensors of the pain pathway Adrienne E. Dubin, Ardem Patapoutian J Clin Invest. 2010;120(11):3760-3772. https://doi.org/10.1172/JCI42843. Review Series Specialized peripheral sensory neurons known as nociceptors alert us to potentially damaging stimuli at the skin by detecting extremes in temperature and pressure and injury-related chemicals, and transducing these stimuli into long- ranging electrical signals that are relayed to higher brain centers. The activation of functionally distinct cutaneous nociceptor populations and the processing of information they convey provide a rich diversity of pain qualities. Current work in this field is providing researchers with a more thorough understanding of nociceptor cell biology at molecular and systems levels and insight that will allow the targeted design of novel pain therapeutics. Find the latest version: https://jci.me/42843/pdf Review series Nociceptors: the sensors of the pain pathway Adrienne E. Dubin1 and Ardem Patapoutian1,2 1Department of Cell Biology, The Scripps Research Institute, La Jolla, California, USA. 2Genomics Institute of the Novartis Research Foundation, San Diego, California, USA. Specialized peripheral sensory neurons known as nociceptors alert us to potentially damaging stimuli at the skin by detecting extremes in temperature and pressure and injury-related chemicals, and transducing these stimuli into long-ranging electrical signals that are relayed to higher brain centers. The activation of functionally distinct cutaneous nociceptor populations and the processing of information they convey provide a rich diversity of pain qualities. Current work in this field is providing researchers with a more thorough understanding of nociceptor cell biology at molecular and systems levels and insight that will allow the targeted design of novel pain therapeutics. -

Potassium Channels in Epilepsy
Downloaded from http://perspectivesinmedicine.cshlp.org/ on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press Potassium Channels in Epilepsy Ru¨diger Ko¨hling and Jakob Wolfart Oscar Langendorff Institute of Physiology, University of Rostock, Rostock 18057, Germany Correspondence: [email protected] This review attempts to give a concise and up-to-date overview on the role of potassium channels in epilepsies. Their role can be defined from a genetic perspective, focusing on variants and de novo mutations identified in genetic studies or animal models with targeted, specific mutations in genes coding for a member of the large potassium channel family. In these genetic studies, a demonstrated functional link to hyperexcitability often remains elusive. However, their role can also be defined from a functional perspective, based on dy- namic, aggravating, or adaptive transcriptional and posttranslational alterations. In these cases, it often remains elusive whether the alteration is causal or merely incidental. With 80 potassium channel types, of which 10% are known to be associated with epilepsies (in humans) or a seizure phenotype (in animals), if genetically mutated, a comprehensive review is a challenging endeavor. This goal may seem all the more ambitious once the data on posttranslational alterations, found both in human tissue from epilepsy patients and in chronic or acute animal models, are included. We therefore summarize the literature, and expand only on key findings, particularly regarding functional alterations found in patient brain tissue and chronic animal models. INTRODUCTION TO POTASSIUM evolutionary appearance of voltage-gated so- CHANNELS dium (Nav)andcalcium (Cav)channels, Kchan- nels are further diversified in relation to their otassium (K) channels are related to epilepsy newer function, namely, keeping neuronal exci- Psyndromes on many different levels, ranging tation within limits (Anderson and Greenberg from direct control of neuronal excitability and 2001; Hille 2001). -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Emerging Roles for Multifunctional Ion Channel Auxiliary Subunits in Cancer T ⁎ Alexander S
Cell Calcium 80 (2019) 125–140 Contents lists available at ScienceDirect Cell Calcium journal homepage: www.elsevier.com/locate/ceca Emerging roles for multifunctional ion channel auxiliary subunits in cancer T ⁎ Alexander S. Hawortha,b, William J. Brackenburya,b, a Department of Biology, University of York, Heslington, York, YO10 5DD, UK b York Biomedical Research Institute, University of York, Heslington, York, YO10 5DD, UK ARTICLE INFO ABSTRACT Keywords: Several superfamilies of plasma membrane channels which regulate transmembrane ion flux have also been Auxiliary subunit shown to regulate a multitude of cellular processes, including proliferation and migration. Ion channels are Cancer typically multimeric complexes consisting of conducting subunits and auxiliary, non-conducting subunits. Calcium channel Auxiliary subunits modulate the function of conducting subunits and have putative non-conducting roles, further Chloride channel expanding the repertoire of cellular processes governed by ion channel complexes to processes such as trans- Potassium channel cellular adhesion and gene transcription. Given this expansive influence of ion channels on cellular behaviour it Sodium channel is perhaps no surprise that aberrant ion channel expression is a common occurrence in cancer. This review will − focus on the conducting and non-conducting roles of the auxiliary subunits of various Ca2+,K+,Na+ and Cl channels and the burgeoning evidence linking such auxiliary subunits to cancer. Several subunits are upregu- lated (e.g. Cavβ,Cavγ) and downregulated (e.g. Kvβ) in cancer, while other subunits have been functionally implicated as oncogenes (e.g. Navβ1,Cavα2δ1) and tumour suppressor genes (e.g. CLCA2, KCNE2, BKγ1) based on in vivo studies. The strengthening link between ion channel auxiliary subunits and cancer has exposed these subunits as potential biomarkers and therapeutic targets. -

Variants in the KCNE1 Or KCNE3 Gene and Risk of Ménière’S Disease: a Meta-Analysis
Journal of Vestibular Research 25 (2015) 211–218 211 DOI 10.3233/VES-160569 IOS Press Variants in the KCNE1 or KCNE3 gene and risk of Ménière’s disease: A meta-analysis Yuan-Jun Li, Zhan-Guo Jin and Xian-Rong Xu∗ The Center of Clinical Aviation Medicine, General Hospital of Air Force, Beijing, China Received 1 August 2015 Accepted 8 December 2015 Abstract. BACKGROUND: Ménière’s disease (MD) is defined as an idiopathic disorder of the inner ear characterized by the triad of tinnitus, vertigo, and sensorineural hearing loss. Although many studies have evaluated the association between variants in the KCNE1 or KCNE3 gene and MD risk, debates still exist. OBJECTIVE: Our aim is to evaluate the association between KCNE gene variants, including KCNE1 rs1805127 and KCNE3 rs2270676, and the risk of MD by a systematic review. METHODS: We searched the literature in PubMed, SCOPUS and EMBASE through May 2015. We calculated pooled odds ra- tios (OR) and 95% confidence intervals (CIs) using a fixed-effects model or a random-effects model for the risk to MD associated with different KCNE gene variants. The heterogeneity assumption decided the effect model. RESULTS: A total of three relevant studies, with 302 MD cases and 515 controls, were included in this meta-analysis. The results indicated that neither the KCNE1 rs1805127 variant (for G vs. A: OR = 0.724, 95%CI 0.320, 1.638, P = 0.438), nor the KCNE3 rs2270676 variant (for T vs. C: OR = 0.714, 95%CI 0.327, 1.559, P = 0.398) was associated with MD risk. -

Circular RNA Hsa Circ 0005114‑Mir‑142‑3P/Mir‑590‑5P‑ Adenomatous
ONCOLOGY LETTERS 21: 58, 2021 Circular RNA hsa_circ_0005114‑miR‑142‑3p/miR‑590‑5p‑ adenomatous polyposis coli protein axis as a potential target for treatment of glioma BO WEI1*, LE WANG2* and JINGWEI ZHAO1 1Department of Neurosurgery, China‑Japan Union Hospital of Jilin University, Changchun, Jilin 130033; 2Department of Ophthalmology, The First Hospital of Jilin University, Jilin University, Changchun, Jilin 130021, P.R. China Received September 12, 2019; Accepted October 22, 2020 DOI: 10.3892/ol.2020.12320 Abstract. Glioma is the most common type of brain tumor APC expression with a good overall survival rate. UALCAN and is associated with a high mortality rate. Despite recent analysis using TCGA data of glioblastoma multiforme and the advances in treatment options, the overall prognosis in patients GSE25632 and GSE103229 microarray datasets showed that with glioma remains poor. Studies have suggested that circular hsa‑miR‑142‑3p/hsa‑miR‑590‑5p was upregulated and APC (circ)RNAs serve important roles in the development and was downregulated. Thus, hsa‑miR‑142‑3p/hsa‑miR‑590‑5p‑ progression of glioma and may have potential as therapeutic APC‑related circ/ceRNA axes may be important in glioma, targets. However, the expression profiles of circRNAs and their and hsa_circ_0005114 interacted with both of these miRNAs. functions in glioma have rarely been studied. The present study Functional analysis showed that hsa_circ_0005114 was aimed to screen differentially expressed circRNAs (DECs) involved in insulin secretion, while APC was associated with between glioma and normal brain tissues using sequencing the Wnt signaling pathway. In conclusion, hsa_circ_0005114‑ data collected from the Gene Expression Omnibus database miR‑142‑3p/miR‑590‑5p‑APC ceRNA axes may be potential (GSE86202 and GSE92322 datasets) and explain their mecha‑ targets for the treatment of glioma. -

Non-Coding Rnas in the Cardiac Action Potential and Their Impact on Arrhythmogenic Cardiac Diseases
Review Non-Coding RNAs in the Cardiac Action Potential and Their Impact on Arrhythmogenic Cardiac Diseases Estefania Lozano-Velasco 1,2 , Amelia Aranega 1,2 and Diego Franco 1,2,* 1 Cardiovascular Development Group, Department of Experimental Biology, University of Jaén, 23071 Jaén, Spain; [email protected] (E.L.-V.); [email protected] (A.A.) 2 Fundación Medina, 18016 Granada, Spain * Correspondence: [email protected] Abstract: Cardiac arrhythmias are prevalent among humans across all age ranges, affecting millions of people worldwide. While cardiac arrhythmias vary widely in their clinical presentation, they possess shared complex electrophysiologic properties at cellular level that have not been fully studied. Over the last decade, our current understanding of the functional roles of non-coding RNAs have progressively increased. microRNAs represent the most studied type of small ncRNAs and it has been demonstrated that miRNAs play essential roles in multiple biological contexts, including normal development and diseases. In this review, we provide a comprehensive analysis of the functional contribution of non-coding RNAs, primarily microRNAs, to the normal configuration of the cardiac action potential, as well as their association to distinct types of arrhythmogenic cardiac diseases. Keywords: cardiac arrhythmia; microRNAs; lncRNAs; cardiac action potential Citation: Lozano-Velasco, E.; Aranega, A.; Franco, D. Non-Coding RNAs in the Cardiac Action Potential 1. The Electrical Components of the Adult Heart and Their Impact on Arrhythmogenic The adult heart is a four-chambered organ that propels oxygenated blood to the entire Cardiac Diseases. Hearts 2021, 2, body. It is composed of atrial and ventricular chambers, each of them with distinct left and 307–330. -

Mutant Three-Repeat Tau Expression Initiates Retinal Ganglion Cell Death Through Caspase-2
UC San Diego UC San Diego Previously Published Works Title Mutant three-repeat tau expression initiates retinal ganglion cell death through Caspase-2. Permalink https://escholarship.org/uc/item/4gr7f6tn Authors Ngolab, Jennifer Canchi, Saranya Rasool, Suhail et al. Publication Date 2021-05-01 DOI 10.1016/j.nbd.2021.105277 Peer reviewed eScholarship.org Powered by the California Digital Library University of California HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Neurobiol Manuscript Author Dis. Author manuscript; Manuscript Author available in PMC 2021 August 18. Published in final edited form as: Neurobiol Dis. 2021 May ; 152: 105277. doi:10.1016/j.nbd.2021.105277. Mutant three-repeat tau expression initiates retinal ganglion cell death through Caspase-2 Jennifer Ngolaba, Saranya Canchia,c, Suhail Rasoola,d, Abderrahman Elmaaroufb, Kimberly Thomasb, Floyd Sarsozaa,c, Jennifer Grundmana, Michael Mantea, Jazmin Florioa, Nimisha Nandankara, Shaina Korouria, Wagner Zagob, Eliezer Masliahe, Robert A. Rissmana,c,* aDepartment of Neurosciences, University of California, San Diego, School of Medicine, La Jolla, CA 92093, United States of America bProthena Biosciences, South San Francisco, CA 94080, United States of America cVeterans Affairs San Diego Healthcare System, San Diego, CA 92161, United States of America dAmydis Inc, San Diego, CA 92121, United States of America eDivision of Neuroscience and Laboratory of Neurogenetics, National Institutes on Aging, NIH, Bethesda, MD 20892, United States of America Abstract The microtubule-associated protein tau is implicated in multiple degenerative diseases including retinal diseases such as glaucoma; however, the way tau initiates retinopathy is unclear. Previous retinal assessments in mouse models of tauopathy suggest that mutations in four-repeat (4R) tau are associated with disease-induced retinal dysfunction, while shifting tau isoform ratio to favor three-repeat (3R) tau production enhanced photoreceptor function. -

Goblet Cell LRRC26 Regulates BK Channel Activation and Protects Against Colitis in Mice
Goblet cell LRRC26 regulates BK channel activation and protects against colitis in mice Vivian Gonzalez-Pereza,1, Pedro L. Martinez-Espinosaa, Monica Sala-Rabanala, Nikhil Bharadwaja, Xiao-Ming Xiaa, Albert C. Chena,b, David Alvaradoc, Jenny K. Gustafssonc,d,e, Hongzhen Hua, Matthew A. Ciorbab, and Christopher J. Linglea aDepartment of Anesthesiology, Washington University School of Medicine in St. Louis, St. Louis, MO 63110; bMcKelvey School of Engineering, Washington University in St. Louis, St. Louis, MO 63130; cDepartment of Internal Medicine, Division of Gastroenterology, Washington University School of Medicine in St. Louis, St. Louis, MO 63110; dDepartment of Medical Chemistry and Cell Biology, University of Gothenburg, 405 30 Gothenburg, Sweden; and eDepartment of Physiology, University of Gothenburg, 405 30 Gothenburg, Sweden Edited by Richard W. Aldrich, The University of Texas at Austin, Austin, TX, and approved December 21, 2020 (received for review September 16, 2020) Goblet cells (GCs) are specialized cells of the intestinal epithelium Despite this progress, ionic transport in GCs and its implications contributing critically to mucosal homeostasis. One of the func- in GC physiology is a topic that remains poorly understood. tions of GCs is to produce and secrete MUC2, the mucin that forms Here, we address the role of the Ca2+- and voltage-activated K+ the scaffold of the intestinal mucus layer coating the epithelium channel (BK channel) in GCs. and separates the luminal pathogens and commensal microbiota GCs play two primary roles: One related to the maintenance from the host tissues. Although a variety of ion channels and of the mucosal barrier (reviewed in refs. -
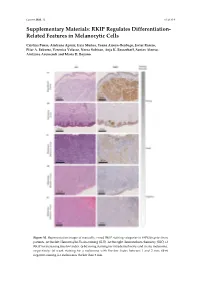
RKIP Regulates Differentiation- Related Features in Melanocytic Cells
Cancers 2020. 12 S1 of S14 Supplementary Materials: RKIP Regulates Differentiation- Related Features in Melanocytic Cells Cristina Penas, Aintzane Apraiz, Iraia Muñoa, Yoana Arroyo-Berdugo, Javier Rasero, Pilar A. Ezkurra, Veronica Velasco, Nerea Subiran, Anja K. Bosserhoff, Santos Alonso, Aintzane Asumendi and Maria D. Boyano Figure S1. Representative images of manually scored RKIP staining categories in FFPE biopsies from patients. At the feft: Hematoxylin-Eosin staining (H-E). At the right: Immunohistochemistry (IHC) of RKIP for increasing Breslow Index. (a-b) Strong staining for intradermal nevus and in situ melanoma, respectively; (c) weak staining for a melanoma with Breslow Index between 1 and 2 mm; (d-e) negative staining for melanomas thicker than 2 mm. Cancers 2020. 12 S2 of S14 Figure S2. RNA Sequencing data analysis. Each row of the figure represents an over-represented gene signature generated by analyzing the up-regulated and down-regulated genes from a differential expression analysis of RKIP Knockdown melanocytes HEMn-LP. Results from the EnrichR Website with a p-value > 0.05. Figure S3. RNA Sequencing data quality. (a) Read counts normalization per sample; (b) Spearman correlation coefficient between replicates; (c) Principal component analysis for replicates. Cancers 2020. 12 S3 of S14 Figure S4. Details of Western Blot from Figure 2 related with RKIP expression in several cell lines. (a) Blots for RKIP and Tubulin in primary and metastatic melanoma and in three primary melanocytes HEMn-DP (dark pigmented), HEMn-LP (light pigmented) and HEM2710. On the right, original blot for each analyzed protein. Highlighted with a red square the information included in the Figure 3 (horizontal rotation). -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

1 Molecular and Genetic Regulation of Pig Pancreatic Islet Cell Development 2 3 4 Seokho Kim1,9, Robert L
bioRxiv preprint doi: https://doi.org/10.1101/717090; this version posted January 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Molecular and genetic regulation of pig pancreatic islet cell development 2 3 4 Seokho Kim1,9, Robert L. Whitener1,9, Heshan Peiris1, Xueying Gu1, Charles A. Chang1, 5 Jonathan Y. Lam1, Joan Camunas-Soler2, Insung Park3, Romina J. Bevacqua1, Krissie Tellez1, 6 Stephen R. Quake2,4, Jonathan R. T. Lakey5, Rita Bottino6, Pablo J. Ross3, Seung K. Kim1,7,8 7 8 1Department of Developmental Biology, Stanford University School of Medicine, 9 Stanford, CA, 94305 USA 10 2Department of Bioengineering, Stanford University, Stanford, CA, 94305 USA 11 3Department of Animal Science, University of California Davis, Davis, CA, 95616 USA 12 4Chan Zuckerberg Biohub, San Francisco, CA 94518, USA. 13 5Department of Surgery, University of California at Irvine, Irvine, CA, 92868 USA 14 6Institute of Cellular Therapeutics, Allegheny Health Network, Pittsburgh, PA, 15212 USA 15 7Department of Medicine, Stanford University School of Medicine, Stanford, CA, 94305 USA 16 8Stanford Diabetes Research Center, Stanford University School of Medicine, 17 Stanford, CA, 94305 USA 18 19 9These authors contributed equally 20 21 Correspondence and requests for materials should be addressed to S.K.K (email: 22 [email protected]) 23 24 Key Words: pancreas; metabolism; organogenesis; b-cell; a-cell; d-cell; diabetes mellitus 25 26 Summary Statement: This study reveals transcriptional, signaling and cellular programs 27 governing pig pancreatic islet development, including striking similarities to human islet 28 ontogeny, providing a novel resource for advancing human islet replacement strategies.