Vaccines That About Vaccines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CLINICAL TRIALS Safety and Immunogenicity of a Nicotine Conjugate Vaccine in Current Smokers
CLINICAL TRIALS Safety and immunogenicity of a nicotine conjugate vaccine in current smokers Immunotherapy is a novel potential treatment for nicotine addiction. The aim of this study was to assess the safety and immunogenicity of a nicotine conjugate vaccine, NicVAX, and its effects on smoking behavior. were recruited for a noncessation treatment study and assigned to 1 of 3 doses of the (68 ؍ Smokers (N nicotine vaccine (50, 100, or 200 g) or placebo. They were injected on days 0, 28, 56, and 182 and monitored for a period of 38 weeks. Results showed that the nicotine vaccine was safe and well tolerated. Vaccine immunogenicity was dose-related (P < .001), with the highest dose eliciting antibody concentrations within the anticipated range of efficacy. There was no evidence of compensatory smoking or precipitation of nicotine withdrawal with the nicotine vaccine. The 30-day abstinence rate was significantly different across with the highest rate of abstinence occurring with 200 g. The nicotine vaccine appears ,(02. ؍ the 4 doses (P to be a promising medication for tobacco dependence. (Clin Pharmacol Ther 2005;78:456-67.) Dorothy K. Hatsukami, PhD, Stephen Rennard, MD, Douglas Jorenby, PhD, Michael Fiore, MD, MPH, Joseph Koopmeiners, Arjen de Vos, MD, PhD, Gary Horwith, MD, and Paul R. Pentel, MD Minneapolis, Minn, Omaha, Neb, Madison, Wis, and Rockville, Md Surveys show that, although about 41% of smokers apy, is about 25% on average.2 Moreover, these per- make a quit attempt each year, less than 5% of smokers centages most likely exaggerate the efficacy of are successful at remaining abstinent for 3 months to a intervention because these trials are typically composed year.1 Smokers seeking available behavioral and phar- of subjects who are highly motivated to quit and who macologic therapies can enhance successful quit rates are free of complicating diagnoses such as depression 2 by 2- to 3-fold over control conditions. -

The Hepatitis B Vaccine Is the Most Widely Used Vaccine in the World, with Over 1 Billion Doses Given
Hepatitis B Vaccine Protect Yourself and Those You Love What is Hepatitis B? Hepatitis B is the most common serious liver infection in the world. It is caused by the hepatitis B virus (HBV), which attacks liver cells and can lead to liver failure, cirrhosis (scarring), or liver cancer later in life. The virus is transmitted through direct contact with infected blood and bodily fluids, and from an infected woman to her newborn at birth. Is there a safe vaccine for hepatitis B? YES! The good news is that there is a safe and effective vaccine for hepatitis B. The vaccine is a series, typically given as three shots over a six-month period that will provide a lifetime of protection. You cannot get hepatitis B from the vaccine – there is no human blood or live virus in the vaccine. The hepatitis B vaccine is the most widely used vaccine in the world, with over 1 billion doses given. The hepatitis B vaccine is the first "anti-cancer" vaccine because it can help prevent liver cancer! Who should be vaccinated against hepatitis B? The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) recommend the hepatitis B vaccine for all newborns and children up to 18 years of age, and all high-risk adults. All infants should receive the first dose of the vaccine in the delivery room or in the first 24 hours of life, preferably within 12 hours. (CDC recommends the first dose within 12 hours vs. the WHO recommendation of 24 hours.) The HBV vaccine is recommended to anyone who is at high risk of infection. -

(ACIP) General Best Guidance for Immunization
8. Altered Immunocompetence Updates This section incorporates general content from the Infectious Diseases Society of America policy statement, 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host (1), to which CDC provided input in November 2011. The evidence supporting this guidance is based on expert opinion and arrived at by consensus. General Principles Altered immunocompetence, a term often used synonymously with immunosuppression, immunodeficiency, and immunocompromise, can be classified as primary or secondary. Primary immunodeficiencies generally are inherited and include conditions defined by an inherent absence or quantitative deficiency of cellular, humoral, or both components that provide immunity. Examples include congenital immunodeficiency diseases such as X- linked agammaglobulinemia, SCID, and chronic granulomatous disease. Secondary immunodeficiency is acquired and is defined by loss or qualitative deficiency in cellular or humoral immune components that occurs as a result of a disease process or its therapy. Examples of secondary immunodeficiency include HIV infection, hematopoietic malignancies, treatment with radiation, and treatment with immunosuppressive drugs. The degree to which immunosuppressive drugs cause clinically significant immunodeficiency generally is dose related and varies by drug. Primary and secondary immunodeficiencies might include a combination of deficits in both cellular and humoral immunity. Certain conditions like asplenia and chronic renal disease also can cause altered immunocompetence. Determination of altered immunocompetence is important to the vaccine provider because incidence or severity of some vaccine-preventable diseases is higher in persons with altered immunocompetence; therefore, certain vaccines (e.g., inactivated influenza vaccine, pneumococcal vaccines) are recommended specifically for persons with these diseases (2,3). Administration of live vaccines might need to be deferred until immune function has improved. -

Immunization Policies and Procedures Manual
Immunization Policies and Procedures Manual Louisiana Department of Health Office of Public Health Immunization Program Revised September 2017 i Center for Community and Preventive Health Bureau of Infectious Diseases Immunization Program TABLE OF CONTENTS I. POLICY AND GENERAL CLINIC POLICY ............................................................................................................................. 1 PURPOSE ........................................................................................................................................................................................... 1 POLICY ON CLINIC SCHEDULING ............................................................................................................................................ 2 POLICY ON PUBLICITY FOR IMMUNIZATION ACTIVITIES .............................................................................................. 4 POLICY ON EDUCATIONAL ACTIVITIES (HEALTH EDUCATION IN IMMUNIZATION CLINICS) .......................... 5 POLICY ON CHECKING IMMUNIZATION STATUS OF ALL CHILDREN RECEIVING SERVICES THROUGH THE HEALTH DEPARTMENT ...................................................................................................................................................... 6 POLICY ON MAXIMIZING TIME SPENT WITH PARENTS DURING IMMUNIZATION CLINICS ............................... 7 POLICY ON ASSISTANCE TO FOREIGN TRAVELERS .......................................................................................................... 9 II. POLICY -

UNICEF Immunization Roadmap 2018-2030
UNICEF IMMUNIZATION ROADMAP 2018–2030 Cover: ©UNICEF/UN065768/Khouder Al-Issa A health worker vaccinates 3-year-old Rahaf in Tareek Albab neighborhood in the eastern part of Aleppo city. UNICEF IMMUNIZATION ROADMAP 2018–2030 Photograph credits: Pages vi-vii: © Shutterstock/thi Page 5: © UNICEF/UN060913/Al-Issa Page 6: © UNICEF/UNI41364/Estey Page 10: © UNICEF/UN061432/Dejongh Page 14: © UNICEF/UNI76541/Holmes Page 18: © UNICEF/UN0125829/Sharma Page 24: © UNICEF/UN059884/Zar Mon Page 26: © UNICEF/UN065770/Al-Issa Page 33: © UNICEF/UN0143438/Alhariri Page 34: © UNICEF/UNI45693/Estey Page 38: © UNICEF/UNI77040/Holmes Page 40: © UNICEF/UN0125861/Sharma Page 44: © UNICEF/UNI41211/Holmes © United Nations Children’s Fund (UNICEF), September 2018 Permission is required to reproduce any part of this publication. Permission will be freely granted to educational or non-profit organizations. Please contact: Health Section, Immunization Team UNICEF 3 United Nations Plaza New York, NY 100017 Contents Abbreviations viii Executive summary 1 1. Introduction 7 1.1 Background 7 1.2 Developing the Roadmap 9 2. Key considerations informing the UNICEF Immunization Roadmap 11 2.1 Key drivers of immunization through 2030 11 2.2 Evolving immunization partnerships 15 2.3 UNICEF’s comparative advantage in immunization 16 3. What is new in this Roadmap? 19 3.1 Immunization coverage as a tracer indicator of child equity 19 3.2 Key areas of work 21 4. Roadmap programming framework 27 4.1 Vision and impact statements 27 4.2 Programming principles 27 4.3 Context-driven responses 27 4.4 Objectives and priorities 29 4.5 Populations and platforms 32 4.6 Country-level immunization strategies 32 5. -
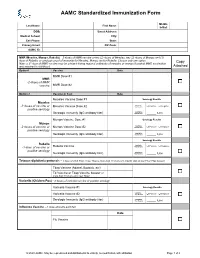
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -

Immunogenicity and Safety of a Third Dose, and Immune Persistence Of
medRxiv preprint doi: https://doi.org/10.1101/2021.07.23.21261026; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Immunogenicity and safety of a third dose, and immune persistence of 2 CoronaVac vaccine in healthy adults aged 18-59 years: interim results 3 from a double-blind, randomized, placebo-controlled phase 2 clinical 4 trial 5 6 Hongxing Pan MSc1*, Qianhui Wu MPH2*, Gang Zeng Ph.D.3*, Juan Yang Ph.D.1, Deyu 7 Jiang MSc4, Xiaowei Deng MSc2, Kai Chu MSc1, Wen Zheng BSc2, Fengcai Zhu M.D.5†, 8 Hongjie Yu M.D. Ph.D.2,6,7†, Weidong Yin MBA8† 9 10 Affiliations 11 1. Vaccine Evaluation Institute, Jiangsu Provincial Center for Disease Control and 12 Prevention, Nanjing, China 13 2. School of Public Health, Fudan University, Key Laboratory of Public Health Safety, 14 Ministry of Education, Shanghai, China 15 3. Clinical Research Department, Sinovac Biotech Co., Ltd., Beijing, China 16 4. Covid-19 Vaccine Department, Sinovac Life Sciences Co., Ltd., Beijing, China 17 5. Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China 18 6. Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, 19 Shanghai, China 20 7. Department of Infectious Diseases, Huashan Hospital, Fudan University, 21 Shanghai, China 22 8. Sinovac Biotech Co., Ltd., Beijing, China NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

The Mississippi Covid-19 Vaccine Confidence Survey: Population Results Report
MAY 2021 THE MISSISSIPPI COVID-19 VACCINE CONFIDENCE SURVEY: POPULATION RESULTS Report A collaborative population-based study The Mississippi Community Engagement Alliance Against COVID-19 Disparities (CEAL) Team The Mississippi State Department of Health: Office of Preventive Health and Health Equity EXECUTIVE SUMMARY Since the Spring of 2020, the Novel 2019 Office of Preventive Health and Health Coronavirus (COVID-19) Pandemic has Equity (OPHHE) disseminated a statewide impacted Mississippians of every race, vaccine confidence survey beginning end ethnicity, age, gender, and income bracket. of December 2020 and collecting data Unfortunately, it has disproportionately until March 2021. The survey is intended to impacted Mississippians of color, the elderly, be representative of Mississippians, with and those living with chronic disease. For intentional efforts invested to reach lower most of the past year, the State has worked income and rural Mississippi populations, as to protect its population through preventive well as the state’s Black, Hispanic (Latino/ measures such as social distancing and Latinx), Asian (including the Vietnamese personal protective equipment. However, population of the Gulf Coast), and Native with the release of COVID-19 Vaccines to American/Choctaw communities. The sur- the public, the population of Mississippi has vey was administered in three languages- the opportunity to embrace a long-term English, Spanish, and Vietnamese- through a solution to COVID-19. That is, if Mississippians mixed-modal survey effort, including: web- are willing to receive the vaccine. To based, paper-based, and verbal-oratory assess Mississippians’ COVID-19 Vaccine administration. All targeted populations confidence, the Mississippi Community were ultimately reached and are represented Engagement Alliance Against COVID-19 in the over 11,000 completed responses Disparities (CEAL) Team and the Mississippi from all 82 of Mississippi’s counties. -

Immunity and How Vaccines Work
Immunity and how vaccines work Dr Brenda Corcoran National Immunisation Office Presentation Outline An understanding of the following principles: • Overview of immunity • Different types of vaccines and vaccine contents • Vaccine failures • Time intervals between vaccine doses • Vaccine overload • Adverse reactions • Herd immunity Immunity Immunity • The ability of the human body to protect itself from infectious disease The immune system • Cells with a protective function in the – bone marrow – thymus – lymphatic system of ducts and nodes – spleen –blood Types of immunity Source: http://en.wikipedia.org/wiki/Immunological_memory Natural (innate) immunity Non-specific mechanisms – Physical barriers • skin and mucous membranes – Chemical barriers • gastric and digestive enzymes – Cellular and protein secretions • phagocytes, macrophages, complement system ** No “memory” of protection exists afterwards ** Passive immunity – adaptive mechanisms Natural • maternal transfer of antibodies to infant via placenta Artificial • administration of pre- formed substance to provide immediate but short-term protection (antitoxin, antibodies) Protection is temporary and wanes with time (usually few months) Active immunity – adaptive mechanisms Natural • following contact with organism Artificial • administration of agent to stimulate immune response (immunisation) Acquired through contact with an micro-organism Protection produced by individual’s own immune system Protection often life-long but may need boosting How vaccines work • Induce active immunity – Immunity and immunologic memory similar to natural infection but without risk of disease • Immunological memory allows – Rapid recognition and response to pathogen – Prevent or modify effect of disease Live attenuated vaccines Weakened viruses /bacteria – Achieved by growing numerous generations in laboratory – Produces long lasting immune response after one or two doses – Stimulates immune system to react as it does to natural infection – Can cause mild form of the disease (e.g.