Technical Report 2016
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Parasitosis in Wild Felids of India: an Overview
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 August 2015 | 7(10): 7641–7648 Review Parasitosis in wild felids of India: an overview Aman Dev Moudgil 1, Lachhman Das Singla 2 & Pallavi 3 ISSN 0974-7907 (Online) 1,2 Department of Veterinary Parasitology, College of Veterinary Science, GADVASU, Ludhiana, Punjab 141004, India ISSN 0974-7893 (Print) 3 School of Public Health and Zoonoses, GADVASU, Ludhiana, Punjab 141004, India 1 [email protected], 2 [email protected] (corresponding author), 3 [email protected] OPEN ACCESS Abstract: Being a tropical country, India provides an ideal environment for the development of parasites as well as for vector populations resulting in a high degree of parasitism in animals and humans. But only a few detailed studies and sporadic case reports are available on the prevalence of parasites in captive wild animals, and the knowledge of parasites and parasitic diseases in wild animals is still in its infancy. The family felidae comprises the subfamily felinae and pantherinae, and within those are all large and small cats. Most of the available reports on parasites in felids describe helminthic infections, which caused morbidities and occasional mortalities in the infected animals. The parasites most frequently found include the nematodes Toxocara, Toxascaris, Baylisascaris, Strongyloides, Gnathostoma, Dirofilaria and Galonchus, the trematode Paragonimus and the cestodes Echinococcus and Taenia. Almost all the studies identified the parasitic stages by classical parasitological techniques and only a few new studies confirmed the species using molecular techniques. Amongst the protozoan parasitic infections reported in felids: babesiosis, trypanosomiasis and coccidiosis are most commonly found. -

SCIENCE CHINA How the White Tiger Lost Its Color, but Kept Its Stripes
SCIENCE CHINA Life Sciences • INSIGHT • October 2014 Vol.57 No.10: 1041–1043 doi: 10.1007/s11427-014-4697-z How the white tiger lost its color, but kept its stripes XU Xiao & LUO Shu-Jin* College of Life Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China Received May 28, 2014; accepted June 9, 2014; published online June 27, 2014 Citation: Xu X, Luo SJ. How the white tiger lost its color, but kept its stripes. Sci China Life Sci, 2014, 57: 1041–1043, doi: 10.1007/s11427-014-4697-z The fantastic range of animal coloration is produced by on the resemblance of white tigers to the chinchilla variant pigment particles of colored material or microscopic struc- in mice, Robinson [1] postulated that white tigers arose be- tures in scales, bristles or feathers that give rise to brilliant cause of a recessive allele at the chinchilla locus (now iridescent colors. In mammals, except for a few exceptions known as the tyrosinase gene). involving nano- or micro-scale structural color (e.g., the Xu et al. [2] tested Robinson’s hypothesis by sequencing blue faces of male baboons), fur color is determined by the the TYR (tyrosinase) gene along with other key pigment melanin. Melanin is synthesized and stored in coat-color-associated genes MC1R (melanocortin 1 recep- melanosomes, a lysosomal organelle of melanocyte, and tor), ASIP (agouti signaling protein), TYRP1 (tyrosi- transferred to adjacent keratinocytes and hairs. There are nase-related protein 1) and SLC7A11 (solute carrier family two types of melanin: eumelanin (black to brown) and 7, member 11) in both white and orange tigers. -

Clinical Cysticercosis: Diagnosis and Treatment 11 2
WHO/FAO/OIE Guidelines for the surveillance, prevention and control of taeniosis/cysticercosis Editor: K.D. Murrell Associate Editors: P. Dorny A. Flisser S. Geerts N.C. Kyvsgaard D.P. McManus T.E. Nash Z.S. Pawlowski • Etiology • Taeniosis in humans • Cysticercosis in animals and humans • Biology and systematics • Epidemiology and geographical distribution • Diagnosis and treatment in humans • Detection in cattle and swine • Surveillance • Prevention • Control • Methods All OIE (World Organisation for Animal Health) publications are protected by international copyright law. Extracts may be copied, reproduced, translated, adapted or published in journals, documents, books, electronic media and any other medium destined for the public, for information, educational or commercial purposes, provided prior written permission has been granted by the OIE. The designations and denominations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the OIE concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries. The views expressed in signed articles are solely the responsibility of the authors. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by the OIE in preference to others of a similar nature that are not mentioned. –––––––––– The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations, the World Health Organization or the World Organisation for Animal Health concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Specific Identification of a Taeniid Cestode from Snow Leopard, Uncia Uncia Schreber, 1776 (Felidae) in Mongolia
Mongolian .Jo~lrnalofBiological Sciences 2003 &)I. ](I): 21-25 Specific Identification of a Taeniid Cestode from Snow Leopard, Uncia uncia Schreber, 1776 (Felidae) in Mongolia Sumiya Ganzorig*?**,Yuzaburo Oku**, Munehiro Okamoto***, and Masao Kamiya** *Department ofZoolopy, Faculty of Biology, National University of Mongol~a,Ulaanbaatar 21 0646, Mongolia **Laboratory of'Parasitology, Graduate School of Veterinary Medicine, Hokkardo University, Sapporo 060- 0818, Japan e-mail: sganzorig(4yahoo.com ***Department of Laboratory Animal Sciences, Tottori University, Tottori 680-8533, Japan Abstract An unknown taeniid cestode, resembling Taenia hydatigena, was recovered from a snow leopard, Uncia uncia in Mongolia. Morphology and nucleotide sequence of the mitochondrial cytochromec oxidase subunit 1gene (mt DNA COI) ofthe cestode found was examined. The cestode is differed from T hydatigena both morphologically and genetically. The differences between two species were in the gross length, different number of testes, presence of vaginal sphincter and in egg size. The nucleotide sequence of this cestode differed from that of 7: hydatigena at 34 of the 384 (8.6%) nucleotide positions examined. The present cestode is very close to 7: kotlani in morphology and size of rostellar hooks. However, the adult stages of the latter species are unknown, and further comparison was unfeasible. Key words: Mongolia, snow leopard, Taenia, taxonomy, mt DNA, cestode, Taeniidae Introduction the use of more than one character for specific identification (Edwards & Herbert, 198 1 ). The snow leopard, Uncia uncia Schreber, 1776 Identification based on hook morphology and (Felidae) is an endangered species within Mongolia measurements are difficult because of overlap in and throughout its range. It is listed in the IUCN the hook lengths between different species. -

TAENIA SOLIUM TAENIASIS/CYSTICERCOSIS DIAGNOSTIC TOOLS REPORT of a STAKEHOLDER MEETING Geneva, 17–18 December 2015
TAENIA SOLIUM TAENIASIS/CYSTICERCOSIS DIAGNOSTIC TOOLS REPORT OF A STAKEHOLDER MEETING Geneva, 17–18 December 2015 Cover_Taeniasis_diagnostic_tools.indd 1 19/05/2016 13:10:59 Photo cover: Véronique Dermauw Cover_Taeniasis_diagnostic_tools.indd 2 19/05/2016 13:10:59 TAENIA SOLIUM TAENIASIS/CYSTICERCOSIS DIAGNOSTIC TOOLS REPORT OF A STAKEHOLDER MEETING Geneva, 17–18 December 2015 TTaeniasis_diagnostic_tools.inddaeniasis_diagnostic_tools.indd 1 119/05/20169/05/2016 113:09:553:09:55 WHO Library Cataloguing-in-Publication Data Taenia Solium Taeniasis/cysticercosis diagnostic tools. Report of a stakeholder meeting, Geneva, 17–18 December 2015 I.World Health Organization. ISBN 978 92 4 1510151 6 Subject headings are available from WHO institutional repository © World Health Organization 2016 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribu- tion –should be addressed to WHO Press through the WHO website (www.who.int/about/licensing/copyright_form/en/ index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. -

Feasibility Study on Opium Licensing in Afghanistan
FEASIBILITY STUDY ON OPIUM LICENSING IN AFGHANISTAN FOR THE PRODUCTION OF MORPHINE AND OTHER ESSENTIAL MEDICINES ﻣﻄﺎﻟﻌﻪ اﻣﮑﺎﻧﺎت در ﻣﻮرد ﺟﻮاز دهﯽ ﺗﺮﻳﺎک در اﻓﻐﺎﻧﺴﺘﺎن ﺑﺮای ﺗﻮﻟﻴﺪ ﻣﻮرﻓﻴﻦ و ادوﻳﻪ ﺟﺎت ﺿﺮوری دﻳﮕﺮ Initial Findings – September 2005 Kabul, Afghanistan The British Institute of International and Comparative Law Hugo Warner • University of Calgary Peter Facchini - Jill Hagel University of Ghent Brice De Ruyver - Laurens van Puyenbroeck University of Kabul Abdul Aziz Ali Ahmad - Osman Babury Cheragh Ali Cheragh - Mohammad Yasin Mohsini University of Lisbon Vitalino Canas - Nuno Aureliano • Shruti Patel • University of Toronto Benedikt Fischer Todd Culbert - Juergen Rehm • Wageningen University Jules Bos - Suzanne Pegge • Ali Wardak • The Senlis Council Gabrielle Archer - Juan Arjona - Luke Bryant Marc Das Gupta - Furkat Elmirzaev - Guillaume Fournier Jane Francis - Thalia Ioannidou - Ernestien Jensema Manna Kamio Badiella - Jorrit Kamminga - Fabrice Pothier Emmanuel Reinert - David Spivack - Daniel Werb FEASIBILITY STUDY ON OPIUM LICENSING IN AFGHANISTAN FOR THE PRODUCTION OF MORPHINE AND OTHER ESSENTIAL MEDICINES Initial Findings – September 2005 Kabul, Afghanistan Study Commissioned by The Senlis Council Study Edited and coordinated by David Spivack Editorial team: Juan Arjona, Jane Francis, Thalia Ioannidou, Ernestien Jensema, Manna Kamio Badiella, Fabrice Pothier. Published 2005 by MF Publishing Ltd 17 Queen Anne’s Gate, London SW1H 9BU, UK ISBN: 0-9550798-2-9 Printed and bound in Afghanistan by Jehoon; Printing Press Other publications -

Nag River Confluence with River Kanhan to NIT Colony, Nagpur (58.7Km) SURVEY PERIOD: 31 JUL 2016 to 30 SEP 2016
Final Feasibility Report National Waterways-72, Region V - Nag River Confluence with River Kanhan to NIT Colony, Nagpur (58.7km) SURVEY PERIOD: 31 JUL 2016 to 30 SEP 2016 Volume - I Prepared for: Inland Waterways Authority of India (Ministry of Shipping, Govt. of India) A-13, Sector – 1, NOIDA Distt. Gautam Budh Nagar, Uttar Pradesh – 201 301 Document Distribution Date Revision Distribution Hard Copy Soft Copy INLAND WATERWAYS 05 Dec 2016 Rev – 0 01 01 AUTHORITY OF INDIA INLAND WATERWAYS 13 Jan 2017 Rev – 1.0 01 01 AUTHORITY OF INDIA INLAND WATERWAYS 17 Oct 2017 Rev – 1.1 04 04 AUTHORITY OF INDIA INLAND WATERWAYS 23 Nov 2017 Rev – 1.2 01 01 AUTHORITY OF INDIA INLAND WATERWAYS 22 Oct 2018 Rev – 1.3 04 04 AUTHORITY OF INDIA ACKNOWLEDGEMENT IIC Technologies Ltd. expresses its sincere gratitude to IWAI for awarding the work of carrying out detailed hydrographic surveys in the New National Waterways in NW-72 in Region V – Nag River from confluence with river Kanhan near Sawangi village to Bridge near NIT Colony, Nagpur. We would like to use this opportunity to pen down our profound gratitude and appreciations to Shri Pravir Pandey, IA&AS, Chairman IWAI for spending his valuable time and guidance for completing this Project. IIC Technologies Ltd., would also like to thank, Shri Alok Ranjan, ICAS, Member (Finance), Shri Shashi Bhushan Shukla, Member (Traffic), Shri S.K. Gangwar, Member (Technical) for their valuable support during the execution of project. IIC Technologies Ltd, wishes to express their gratitude to Capt. Ashish Arya, Hydrographic Chief IWAI, Cdr. -

TRAFFIC Post, India Office Newsletter (PDF)
• South Asia unites to curb illegal • India ranks highest in Tiger parts Pg 8 trade in endangered wildlife seizure over last decade • Officers from Uttar Pradesh, Pg 3 Maharashtra, Andhra Pradesh and West Bengal sharpen skills on wildlife law enforcement • Raja and Jackie: The new ATE champions fighting wildlife Pg 3 crime • World leaders echo support to IN FOCUS ensure doubling of world's wild Pg 4 India TRAFFIC © Tiger population • Efforts augmented to ensure sustainable harvesting and trade Pg 4 TRAFFIC Alert (Latest news on of MAPs illegal wildlife trade in India): Pg 5 • TRAFFIC India's film “Don't Buy T Trouble” now available in Hindi • Guard held with zebra skin Pg 5 TRAFFIC INDIA UPD • Customs officials seize Pg 6 ornamental fish at Coimbatore Airport • Five tonnes of Red Sanders logs Pg 7 • Experts link up to combat illegal Pg 5 seized at Gujarat port wildlife trade in Sri Lanka TRAFFIC ALER • Four tonnes of Sea cucumber Pg 7 seized in Tamil Nadu • Email alerts on CITES related Pg 6 SIGNPOST: Other significant Pg 12 OUTPOST issues now available by subscription news stories to read SIGNPOST Pg 10 NEW SECTION WILD CRY : Illegal wildlife trade threatens the future of many species in the © Ola Jennersten Ola © wild. This section highlights the plight of CITES one such species in trade. UPDATE • Tiger killers will be brought to Pg 6 book, says CITES Secretary General Pangolins in peril TRAFFIC POST march 2011 South Asia unites to curb illegal trade in endangered wildlife he eight countries of South Asia—India, Nepal, Pakistan, TAfghanistan, Bangladesh, Bhutan, Maldives and Sri Lanka— joined forces and established the South Asia Wildlife Enforcement Network (SAWEN) to collaborate and co-operate on strengthening wildlife law enforcement in the region. -
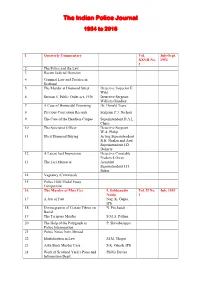
Index of the Indian Police Journal Issues from the Year 1954 to 2016
The Indian Police Journal 1954 to 2016 1 Quarterly Commentary Vol. July-Sept. XXVII No. 1954 3 2 The Police and the Law 3 Recent Judicial Decision 4 Criminal Law and Practice in Scotland 5 The Murder at Diamond Street Detective Inspector E. Wild 6 Section 5, Public Order act, 1936 Detective Sergeant William Grindley 7 A Case of Homicidal Poisoning Dr. Donald Teare 8 Previous Conviction Records Sergeant P.J. Nichols 9 The Case of the Headless Corpse Superintendent D.A.L. Chase 10 The Specialist Officer Detective Sergeant W.A. Philip 11 Illicit Diamond Buying Acting Superintendent B.H. Nealan and Asst. Superintendent J.D. Doherty 12 A Latent heel Impression Detective Constable Frederick Owen 13 The Lari Massacre Assistant Superintendent J.H. Baker 14 Vagrancy (Continued) 15 Police Gold Medal Essay Competition 16 The Murder of Miss Cox I. Sobhanadri Vol. II No. July 1955 Naidu 1 17 A Jest of Fate Nag. K. Gupta, IPS 18 Disintegration of Certain Fibres on N. Pitchandi Burial 19 The Tarapore Murder S.M.A. Pathan 20 The Help of the Polygraph in P. Shivabasappa Police Interrogation 21 Police Notes from Abroad 22 Identification in Law M.M. Thapar 23 Aska Bank Murder Case S.K. Ghosh, IPS 24 Work of Scotland Yard‘s Press and Phillis Davies Information Deptt. 25 Murder or Accident L. Forstner 26 The Finger Prints of Bahadur Khan Shiam Narain 27 A Chain of Forensic V.R. Kher, I.P. Vol. II No. January Laboratories in India 3 1956 28 Belbad Colliery Dacoity N.S. -

Occurrence of Desert Wheatear Oenanthe Deserti in ICRISAT Campus, Medak District, Andhra Pradesh, India R
Occurrence of Desert Wheatear Oenanthe deserti in ICRISAT Campus, Medak District, Andhra Pradesh, India R. Sreekar1*, Ashwin Naidu1 and C. Srinivasulu2 On 2nd December 2007 at around 1030 hrs, RS and Pittie, A. and M. Shafaat Ulla (2005). Occurrence AN (R. Sreekar and Ashwin Naidu) sighted and of Desert Wheatear Oenanthe deserti and Isabelline photographed (Fig. 1) a chat-sized bird near ICRISAT Wheatear Oenanthe isabellina in Mahbubnagar Lake in ICRISAT Campus (17O53’ N & 78O27’ E), District, Andhra Pradesh. Journal of the Bombay Medak district, Andhra Pradesh, India. The said bird Natural History Society, 102 (2): 234-235. was sighted perching on Phoenix acaulis and had distinctive sandy-brown plumage with pale white ACKNOWLEDGEMENTS supercilium, white rump and black tail with white tips We express our heartfelt thanks to Mr. Rajeev Mathew and edges. The bird landed on a nearby pathway, for helping in identification; Dr. Tom C. Hash of where it was observed for a total of c. 15 minutes ICRISAT for permission to conduct bird surveys. CS before it returned to the perch. The bird flew from is thankful to Head, Department of Zoology, Osmania its perch to an open ground and then returned to the University for permission and encouragements. perch. We had an opportunity to observe the bird for about 15 minutes. The bird perched in an upright stance and had long legs. The bird was identified as Desert WheatearOenanthe deserti following Kazmierczak, (2000) and Grimmett et al. (1998). The identification was also independently confirmed from the photograph by more experienced members of the Birdwatchers’ Society of Andhra Pradesh. -

0 0 101130121812171Masterpl
2 Relocation of State Museum & Zoo, Thrissur 3 Relocation of State Museum & Zoo, Thrissur 4 Relocation of State Museum & Zoo, Thrissur 5 Relocation of State Museum & Zoo, Thrissur 6 Relocation of State Museum & Zoo, Thrissur 7 Relocation of State Museum & Zoo, Thrissur 8 Relocation of State Museum & Zoo, Thrissur Relocation of State Museum & Zoo, Thrissur TABLE OF CONTENTS PART – I Sl. No. Chapter No Subjec t Pag e No 1 Exe cutive summery 1-8 Introduction History of Thrissur Zoo 2 1 9-20 Fe atures of area propose d for new Zoological Park Appraisal of Present Arr angements and 3 2 21-22 constrains PART – II 4 1 Future obje ctive , Miss ion, Vision 23-26 Future Action Plan-themes, captive breeding, Proposed Master Layout, 5 2 27-64 visitor facilities, animal health care, water and electricity supply etc. Personnel Planning 6 3 Propose d Administrative Set up 65-68 Staffing Pattern 7 4 Disast e r Manage ment 69-72 8 5 Contingency Plan 73-78 Capacity building of officers and staff of 9 6 propose d new Zoological Park at 79-82 Puthur 10 7 Financial forecast for imple mentation of 83-84 the Master Plan Action Plan for imple mentation of 11 8 85-96 Master Plan 12 Anne xure - I Propose d staffing pattern 97-100 Propose d collection plan for new 13 Anne xure - II 101-110 Zoological Park at Puthur Pre se nt colle cti on of an im als i n T hri ss ur 14 Anne xure - III Zoo 111-112 List of animals e ndemic to We stern 15 Anne xure - IV 113-116 Ghats Atte ndance project ions and visitor 16 Anne xure - V 117-120 re quire ment G.O (MS) 16 /201 2/F&WLD date d, 24/02/2013 of Government of Kerala 17 Anne xure - VI according approval for establishment of 121-122 ne w Zoological Park and winding up of e xisting Thrissur Zoo Le tter No. -

A Commentary on the April 2015 and February 2015 Country Information and Guidance Reports Issued on India
THIS DOCUMENT SHOULD BE USED AS A TOOL FOR IDENTIFYING RELEVANT COUNTRY OF ORIGIN INFORMATION. IT SHOULD NOT BE SUBMITTED AS EVIDENCE TO THE HOME OFFICE, THE TRIBUNAL OR OTHER DECISION MAKERS IN ASYLUM APPLICATIONS OR APPEALS © Still Human Still Here 2015 6 August 2015 (COI included up to 13 July 2015) A Commentary on the April 2015 and February 2015 Country Information and Guidance reports issued on India This commentary identifies what the ‘Still Human Still Here’ coalition considers to be the main inconsistencies and omissions between the currently available country of origin information (COI) and case law on India and the conclusions reached in the following Country Information and Guidance (CIG) reports issued by the UK Home Office: o Country information and guidance report: Women fearing gender-based harm/violence, India, April 2015 o Country information and guidance report: Background information, including actors of protection, and internal relocation, India, February 2015 Where we believe inconsistencies have been identified, the relevant section of the CIG report is highlighted in blue. An index of full sources of the COI referred to in this commentary is also provided at the end of the document (COI up to 13 July 2015). This commentary is a guide for legal practitioners and decision-makers in respect of the relevant COI, by reference to the sections of the CIG reports on India. The document should be used as a tool to help to identify relevant COI and the COI referred to can be considered by decision makers in assessing asylum applications and appeals. This document should not be submitted as evidence to the UK Home Office, the Tribunal or other decision makers in asylum applications or appeals.