Modified Methods for the Synthesis of Carbazole from Phenothiazine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American Version
COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIV Detection of preservatives and fermentation inhibitors Method OIV-MA-AS4-02E Type IV method Detection of preservatives and fermentation inhibitors Method A 35 modified by resolution Oeno 6/2006 1. Examination of dehydroacetic acid 1.1 Principle Wine acidified with sulfuric acid is extracted with a mixture of equal parts of diethyl ether and petroleum ether. After evaporation of the solvent, the extract, recovered with a small quantity of 96% ethanol (v/v) is deposited on a thin layer of polyamide and silica gel with fluorescent indicator and subjected to the action of the mobile solvent (benzene-acetone-acetic acid). The dehydroacetic acid is identified and characterized by ultraviolet examination of the chromatogram. 1.2 Apparatus 1.2.1 Equipment for thin layer chromatography 1.2.2 Oven 1.2.3 Rotary evaporator 1.2.4 UV lamp 254 nm. 1.3 Reagents 1.3.1 Diethyl ether 1.3.2 Petroleum ether (boiling point 40 °C) 1.3.3 Methanol 1.3.4 Sulfuric acid, 20% (v/v) 1.3.5 Anhydrous sodium sulfate. 1.3.6 Ethanol, 96% (v/v) . 1.3.7 Chromatographic separation layer: 10 g polyamide powder with fluorescent indicator(e.g. polyamide DC II UV254 from Macherey-Nagel) mixed vigorously with 60 mL methanol. Add while stirring, 10 ml of water and 10ml of silica gel (with fluorescent indicator), e.g. Kiesselgel GF254 Merck. Spread this mixture on 5 plates (200 x 200 mm) to a thickness of 0.25 mm. Dry the plates at room temperature for 30 minutes, then place in a 70°C oven for 10 min. -

SDS Contains All of the Information Required by the HPR
SAFETY DATA SHEET Preparation Date: 4/1/2014 Revision date 6/24/2019 Revision Number: G2 1. IDENTIFICATION Product identifier Product code: P1040 Product Name: PETROLEUM ETHER, B.R. 20 DEG -40 DEG C Other means of identification Synonyms: Benzine (light petroleum distillate) Benzoline Canadol Ligroin Painters naphtha Refined solvent naphtha CAS #: 8032-32-4 RTECS # OI6180000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Solvent. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Tom Tyner (USA - West Coast) Contact Person: Ibad Tirmiz (USA - East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Inhalation (Gases) Category 4 Acute toxicity - Inhalation (Vapors) Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 2 Aspiration toxicity Category 1 Flammable liquids Category 2 Label elements Product code: P1040 Product name: PETROLEUM ETHER, Page 1 / 13 B.R. 20 DEG -40 DEG C Danger Hazard statements Harmful if inhaled Causes -

Hydrocarbons 95 % Petroleum Ether Hydrocarbons 95 %
Hydrocarbons 95 % Petroleum Ether Hydrocarbons 95 % Our range of hydrocarbons 95 % offers a wide selec- Hydrocarbons 95 % are used in numerous industrial tion of high-grade quality products, which meet the processes. The applications range from propellant high requirements for components utilised in de- components for plastic foams and cosmetics, pro- manding chemical processes. cess adjuvants and reaction media in the pharma- ceutical, agrochemical and fine chemical industries, The hydrocarbons 95 % product range comprises to specific printing inks and latent heat storage. aliphatic and alicyclic hydrocarbons with a minimum purity of 95 % and covers a chain length ranging Hydrocarbons 95 % are excellently suited as high- from C5 to C16. For more far reaching, process- purity solvents in crystallisation, extraction, HPLC induced quality requirements, please refer to the and SMB chromatography processes. Special low- hydrocarbons 99 % product group. odour qualities have been specifically developed for cosmetic applications. Hydrocarbons 95 % CAS-No. Colour Density Destillation range Evaporation rate Refractive index at 15°C kg/m3 at 101.3 kPa (Ether = 1) nD20 Typical Properties ISO 6271 ISO 12185 ASTM D 1078 DIN 53170 DIN 51423-2 iso-Pentane 78-78-4 < 5 624 27–29 < 1.0 1.354 n-Pentane 109-66-0 < 5 631 36–38 1.0 1.358 n-Hexane 110-54-3 < 5 665 68–70 1.5 1.375 n-Heptane 142-82-5 < 5 688 97–100 3.1 1.388 iso-Octane 540-84-1 < 5 696 98–101 2.9 1.392 (2,2,4-Trimethylpentane) n-Octane 111-65-9 < 5 708 124–127 8.0 1.398 n-Nonane* 111-84-2 < 5 -

Material Safety Data Sheet (Msds/Sds) Was Prepared by Technical Personnel Based on Data That They Believe in Their Good Faith Judgment Is Accurate
Version: 1.0 Revision Date: 11-14-2014 SAFETY DATA SHEET 1. Identification Product identifier: PETROLEUM ETHER Other means of identification Product No.: 6128, H489, 9272, 4983, 4980, 9270, 4976, 9268, 4971, 9265 Recommended use and restriction on use Recommended use: Not available. Restrictions on use: Not known. Manufacturer/Importer/Supplier/Distributor Information Manufacturer Company Name: Avantor Performance Materials, Inc. Address: 3477 Corporate Parkway, Suite 200 Center Valley, PA 18034 Telephone: Customer Service: 855-282-6867 Fax: Contact Person: Environmental Health & Safety e-mail: [email protected] Emergency telephone number: 24 Hour Emergency: 908-859-2151 Chemtrec: 800-424-9300 2. Hazard(s) identification Hazard Classification Physical Hazards Flammable liquids Category 2 Health Hazards Germ Cell Mutagenicity Category 1B Carcinogenicity Category 1B Aspiration Hazard Category 1 Label Elements Hazard Symbol: Signal Word: Danger Hazard Statement: Highly flammable liquid and vapor. May be fatal if swallowed and enters airways. May cause genetic defects. May cause cancer. Precautionary SDS_US - SDS000001091 1/10 Version: 1.0 Revision Date: 11-14-2014 Statement Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Use personal protective equipment as required. Keep away from heat/sparks/open flames/hot surfaces. No smoking. Response: IF exposed or concerned: Get medical advice/attention. IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT induce vomiting. Storage: Store locked up. Store in a well-ventilated place. Keep container tightly closed. Keep cool. Disposal: Dispose of contents/container to an appropriate treatment and disposal facility in accordance with applicable laws and regulations, and product characteristics at time of disposal. -

SUPERCRITICAL CARBON DIOXIDE EXTRACTION of SOUTHERN PINE and PONDEROSA PINE1 David C
SUPERCRITICAL CARBON DIOXIDE EXTRACTION OF SOUTHERN PINE AND PONDEROSA PINE1 David C. Ritter Scientist2 Department of Forest Products University of Minnesota St. Paul, MN 55108 and Alton G. Campbell Associate Professor Department of Forest Products University of Idaho Moscow, ID 83843 (Received November 1989) ABSTRACT Pine wood and bark were extracted with supercritical (SC) carbon dioxide under various experimental conditions. The extractive yields ranged from 20-60% relative to the total diethyl ether extractive content. The yields were dependent on temperature, pressure, particle size, and fluid to wood ratio. The addition of ethanol to bark particles prior to SC CO, extraction produced higher yields of extracts relative to extractions without the addition of ethanol. Gas chromatographic (GC) analysis of selected SC carbon dioxide extracts revealed that the concentration of resin acids, as well as the yield of pure abietic acid, increased with temperature at constant pressure. Fatty acids were more soluble in SC carbon dioxide relative to diethyl ether. The concentration of fatty acids in SC carbon dioxide extracts did not appear to follow definite trends. In addition, observation of the wood particles with scanning electron microscope (SEM) revealed that the supercritical extraction process did not appear to sig- nificantly alter the wood surface structure. Keywords: Southern pine, ponderosa pine, supercritical, carbon dioxide, extraction, scanning electron microscopy. INTRODUCTION Supercritical fluid (SCF) extraction is a rapidly developing technology that has great potential for separating and purifying high value products (Hoyer 1985; Williams 198 1; Schneider 1978). Carbon dioxide (CO,) is probably the most studied supercritical fluid since it is nonflammable, noncorrosive, nontoxic, and inexpensive (Brogle 1982). -
Safety Data Sheet: Petroleum Ether 40-60 °C
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH) Petroleum ether 40-60 °C ROTIPURAN® p.a., ACS, ISO article number: T173 date of compilation: 2021-05-25 Version: 1.1 en Revision: 2021-05-26 Replaces version of: 2021-05-25 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Identification of the substance Petroleum ether 40-60 °C ROTIPURAN® p.a., ACS, ISO Article number T173 Registration number (REACH) not relevant (mixture) 1.2 Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses: Laboratory chemical Laboratory and analytical use Uses advised against: Do not use for products which come into contact with foodstuffs. Do not use for private purposes (household). 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone:+49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data :Department Health, Safety and Environment sheet: e-mail (competent person): [email protected] 1.4 Emergency telephone number Name Street Postal Telephone Website code/city National Poisons Information Beaumont Road Dublin 9 01 809 2166 https:// Centre www.poisons.ie/ Beaumont Hospital SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Section Hazard class Cat- Hazard class and Hazard egory category statement 2.6 Flammable liquid 2 Flam. -
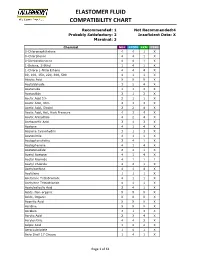
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air, -

[email protected]
SAFETY DATA SHEET Creation Date 11-Feb-2010 Revision Date 18-Jan-2018 Revision Number 4 1. Identification Product Name Petroleum Ether Cat No. : E120-4; E120-4LC; E120SK-4; E120SS-50; E139-1; E139-4; E139-20; E139-200; E139-500; E139FB-19; E139FB-50; E139FB-115; E139FB-200; E139RB-50; E139RB-115; E139RB-200; E139RS-19; E139RS-28; E139RS-50; E139RS-115; E139RS-200; E139S-4; E139SK-4; E139SS-28; E139SS-50; E139SS-200; P480-4; P480-4LC; P480RS-19; P480RS-28; P480RS-50; P480RS-115; P480RS-200; P480SS-28; P480SS-50; P480SS-115; P480SS-200; P481RS-200; P481SS-200 CAS-No 8032-32-4 Synonyms Ligroine; Benzine; Naphtha Petroleum; Naphtha Solvent (Optima/Pesticide/Certified ACS) Recommended Use Laboratory chemicals. Uses advised against Not for food, drug, pesticide or biocidal product use Details of the supplier of the safety data sheet Company Fisher Scientific One Reagent Lane Fair Lawn, NJ 07410 Tel: (201) 796-7100 Emergency Telephone Number CHEMTRECÒ, Inside the USA: 800-424-9300 CHEMTRECÒ, Outside the USA: 001-703-527-3887 2. Hazard(s) identification Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Flammable liquids Category 2 Germ Cell Mutagenicity Category 1B Carcinogenicity Category 1B Aspiration Toxicity Category 1 Label Elements Signal Word Danger Hazard Statements Highly flammable liquid and vapor May be fatal if swallowed and enters airways ______________________________________________________________________________________________ Page 1 / 8 Petroleum Ether Revision Date 18-Jan-2018 ______________________________________________________________________________________________ May cause genetic defects May cause cancer Precautionary Statements Prevention Obtain special instructions before use Do not handle until all safety precautions have been read and understood Use personal protective equipment as required Keep away from heat/sparks/open flames/hot surfaces. -

Carbon Tetrachloride Chloride, the Risk of NHL Was Lower Than in the Earlier Study, Although Still (Nonsignificantly) Elevated (Radicanet Al
Report on Carcinogens, Fourteenth Edition For Table of Contents, see home page: http://ntp.niehs.nih.gov/go/roc Carbon Tetrachloride chloride, the risk of NHL was lower than in the earlier study, although still (nonsignificantly) elevated (Radicanet al. 2008). CAS No. 56-23-5 Properties Reasonably anticipated to be a human carcinogen Carbon tetrachloride is a halomethane that exists at room tempera- First listed in the Second Annual Report on Carcinogens (1981) ture as a clear, colorless, heavy liquid with a sweetish, aromatic, mod- Also known as tetrachloromethane erately strong ethereal odor. It is very slightly soluble in water, soluble in ethanol and acetone, and miscible with benzene, chloroform, ether, Cl carbon disulfide, petroleum ether, and oils. It is nonflammable and Cl C is stable under normal temperatures and pressures (Akron 2009). Cl Cl Physical and chemical properties of carbon tetrachloride are listed Carcinogenicity in the following table. Carbon tetrachloride is reasonably anticipated to be a human car- Property Information cinogen based on sufficient evidence of carcinogenicity from studies Molecular weight 153.8 in experimental animals. Specific gravity 1.594 at 20°C/4°C Cancer Studies in Experimental Animals Melting point –23°C Boiling point 76.8°C Carbon tetrachloride caused tumors in several species of experimen- Log Kow 2.83 tal animals, at two different tissue sites, and by several different routes Water solubility 793 mg/L at 25°C of exposure. It caused benign or malignant liver tumors when admin- Vapor pressure 115 mm Hg at 25°C istered (1) orally in mice and rats of both sexes, in hamsters, and in Vapor density relative to air 5.32 trout, (2) by subcutaneous injection in male rats, and (3) by inhalation Source: HSDB 2009. -

Extraction and Potential of Cinnamon Essential Oil Towards Repellency and Insecticidal Activity
International Journal of Scientific and Research Publications, Volume 4, Issue 7, July 2014 1 ISSN 2250-3153 Extraction and Potential of Cinnamon Essential Oil towards Repellency and Insecticidal Activity Nur Nasulhah Kasim*, Syarifah Nursyimi Azlina Syed Ismail, N.D. Masdar , Fatimah Ab Hamid, W.I Nawawi. Faculty of Applied Sciences, Universiti Teknologi MARA Perlis, 02600 Arau, Perlis, Malaysia Abstract- The potential of Cinnamon essential oil as a natural is known as kurundu which was recorded in English in the 17th insecticides and ants repellent was studied. Cinnamon cassia century as Korunda (Knox and Robert, 2008). In Indonesia, bark was extracted using hydrodistillation and soxhlet extraction where cinnamon is cultivated in Java and Sumatra, it is called method with three different solvents i. e. petroleum ether, hexane kayu manis ("sweet wood") and sometimes cassia vera, the and dichloromethane. All extraction was carried out for 6 hours "real" cassia ( Samat and Bell, 2009). at 1 atm. The highest yield of cinnamon oil was obtained by Repellent is defined by Fried et al. (2007) as substances that soxhlet extraction in dichloromethane, followed by hexane and cause insect to turn away. Repellent have been used to prevent petroleum ether were 5.22 %, 3.84 % and 3.71 %, respectively. insects or specifically in this study, the ants from harming or While only 1.82 % yields of cinnamon essential oil extracted annoying human daily life as they always swarm over host’s when using hydrodistillation method. The volatile compounds of food especially food that have been left on tables or being cinnamon essential oil were identified using GC-MS analysis. -

Investigation Into the Addition of Chloroform and Bromoform to O- Bromobenzaldehyde
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1940 Investigation into the addition of chloroform and bromoform to o- bromobenzaldehyde Leland Marshall Yates The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Yates, Leland Marshall, "Investigation into the addition of chloroform and bromoform to o- bromobenzaldehyde" (1940). Graduate Student Theses, Dissertations, & Professional Papers. 8180. https://scholarworks.umt.edu/etd/8180 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. AH IHVEStlGATIO'^r INTO THE ADDITION OF CHLOROFORîi A33D üEu^OPOEM TO 0-&KO„*03.Jî2ALDEliYDÏÏ by Leland Marshall Yates Submitted in partial fulfillment of The requirements for the Degree of Master of Arts Montana State University August 1940 Approved t Chairman Board of Ihcttminers Chairman of Committee on Graduate Study Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: EP38981 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. -

Separation, Purification and Identification of the Components of a Mixture Supplementary Material
Supplementary information for Comprehensive Organic Chemistry Experiments for the Laboratory Classroom © The Royal Society of Chemistry 2017 Separation, purification and identification of the components of a mixture Supplementary Material Experimental notes Background topics……………………………………………………………………………………..1 Experimental details………..………………………………………………………………………….2 Figures Photos of the experiment…………………………………………………………………….…..…….3 IR spectra…………………………………………………….……………………………………..……5 Experimental notes Background topics This experiment does not involve typical chemical reactions of organic compounds, with the exception of acid-base reactions. Its aim is to provide the students the knowledge of fundamental experimental techniques of unitary operations, such as extraction, distillation, filtration, recrystallization and thin layer chromatography. The work (two laboratory sessions) has a low difficulty level and is adequate to introductory level students, especially engineering students (chemical, biochemical, material sciences, biomedical and related areas) or Chemistry students at introductory level. This simple pedagogic experiment was already realized by over 500 students of the Faculty of Sciences and Technology, Universidade Nova de Lisboa (in classes of 22 students/class, 11 groups of two), who accomplished the work in two (2x3 hours) laboratory sessions. The students must be encouraged to rationalise the principles of the unitary operations performed and to understand their application. If this work is a first experiment in an Organic Chemistry laboratory class, every experimental detail must be extensively discussed. Special attention and care must also be given to collection and treatment of results (calculation of Rf values and recuperation “yields”, measurement of physical properties, e.g. melting points). It is also important to encourage the students to compare the experimental results with those referred in the literature. Hints for the answers to the proposed questions and topics to discussion: 1.