Solvents Application Guide Products, Packaging, and Delivery Systems Table of Contents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American Version
COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIV Detection of preservatives and fermentation inhibitors Method OIV-MA-AS4-02E Type IV method Detection of preservatives and fermentation inhibitors Method A 35 modified by resolution Oeno 6/2006 1. Examination of dehydroacetic acid 1.1 Principle Wine acidified with sulfuric acid is extracted with a mixture of equal parts of diethyl ether and petroleum ether. After evaporation of the solvent, the extract, recovered with a small quantity of 96% ethanol (v/v) is deposited on a thin layer of polyamide and silica gel with fluorescent indicator and subjected to the action of the mobile solvent (benzene-acetone-acetic acid). The dehydroacetic acid is identified and characterized by ultraviolet examination of the chromatogram. 1.2 Apparatus 1.2.1 Equipment for thin layer chromatography 1.2.2 Oven 1.2.3 Rotary evaporator 1.2.4 UV lamp 254 nm. 1.3 Reagents 1.3.1 Diethyl ether 1.3.2 Petroleum ether (boiling point 40 °C) 1.3.3 Methanol 1.3.4 Sulfuric acid, 20% (v/v) 1.3.5 Anhydrous sodium sulfate. 1.3.6 Ethanol, 96% (v/v) . 1.3.7 Chromatographic separation layer: 10 g polyamide powder with fluorescent indicator(e.g. polyamide DC II UV254 from Macherey-Nagel) mixed vigorously with 60 mL methanol. Add while stirring, 10 ml of water and 10ml of silica gel (with fluorescent indicator), e.g. Kiesselgel GF254 Merck. Spread this mixture on 5 plates (200 x 200 mm) to a thickness of 0.25 mm. Dry the plates at room temperature for 30 minutes, then place in a 70°C oven for 10 min. -

SDS Contains All of the Information Required by the HPR
SAFETY DATA SHEET Preparation Date: 4/1/2014 Revision date 6/24/2019 Revision Number: G2 1. IDENTIFICATION Product identifier Product code: P1040 Product Name: PETROLEUM ETHER, B.R. 20 DEG -40 DEG C Other means of identification Synonyms: Benzine (light petroleum distillate) Benzoline Canadol Ligroin Painters naphtha Refined solvent naphtha CAS #: 8032-32-4 RTECS # OI6180000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Solvent. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Tom Tyner (USA - West Coast) Contact Person: Ibad Tirmiz (USA - East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Inhalation (Gases) Category 4 Acute toxicity - Inhalation (Vapors) Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 2 Aspiration toxicity Category 1 Flammable liquids Category 2 Label elements Product code: P1040 Product name: PETROLEUM ETHER, Page 1 / 13 B.R. 20 DEG -40 DEG C Danger Hazard statements Harmful if inhaled Causes -

Toughening Behaviour of Rubber-Modified Thermoplastic Polymers Involving Very Small Rubber Particles: 1
Toughening behaviour of rubber-modified thermoplastic polymers involving very small rubber particles: 1. A criterion for internal rubber cavitation D. Dompas and G. Groeninckx* Catholic University of Leuven, Laboratory of Macrornolecular Structural Chemistry, Celestijnenlaan 20OF, B-3001 Heverlee, Belgium (Received 26 January 1994) The criteria for internal cavitation of rubber particles have been evaluated. It is shown that internal rubber cavitation can be considered as an energy balance between the strain energy relieved by cavitation and the surface energy associated with the generation of a new surface. The model predicts that there exists a critical particle size for cavitation. Very small particles (100-200nm) are not able to cavitate. This critical-particle-size concept explains the decrease in toughening efficiency in different rubber-modified systems involving very small particles. (Keywords: rubber toughening; particle size; rubber cavitation) INTRODUCTION and associated matrix shear yielding is found in rubber-modified PC ~-7, PVC a-x°, poly(butylene tere- Many glassy polymers are brittle. For structural applica- phthalate (PBT) 11, nylon.612-1 s, nylon_6,616 and epoxy tions, this is clearly unwanted and it is well known that systems17 20. The matrix polymers in these rubber- the impact properties can be improved by the incorpora- modified systems are either crosslinked or have a high tion of a dispersed elastomeric phase Lz. The mechanism entanglement density, thus being polymers for which the by which the toughness is enhanced depends on the crazing mechanism is suppressed 21. This is not to say intrinsic ductility of the matrix material and on the that rubber cavitation can only appear in high-entangle- morphology of the blends 3. -

Hydrocarbons 95 % Petroleum Ether Hydrocarbons 95 %
Hydrocarbons 95 % Petroleum Ether Hydrocarbons 95 % Our range of hydrocarbons 95 % offers a wide selec- Hydrocarbons 95 % are used in numerous industrial tion of high-grade quality products, which meet the processes. The applications range from propellant high requirements for components utilised in de- components for plastic foams and cosmetics, pro- manding chemical processes. cess adjuvants and reaction media in the pharma- ceutical, agrochemical and fine chemical industries, The hydrocarbons 95 % product range comprises to specific printing inks and latent heat storage. aliphatic and alicyclic hydrocarbons with a minimum purity of 95 % and covers a chain length ranging Hydrocarbons 95 % are excellently suited as high- from C5 to C16. For more far reaching, process- purity solvents in crystallisation, extraction, HPLC induced quality requirements, please refer to the and SMB chromatography processes. Special low- hydrocarbons 99 % product group. odour qualities have been specifically developed for cosmetic applications. Hydrocarbons 95 % CAS-No. Colour Density Destillation range Evaporation rate Refractive index at 15°C kg/m3 at 101.3 kPa (Ether = 1) nD20 Typical Properties ISO 6271 ISO 12185 ASTM D 1078 DIN 53170 DIN 51423-2 iso-Pentane 78-78-4 < 5 624 27–29 < 1.0 1.354 n-Pentane 109-66-0 < 5 631 36–38 1.0 1.358 n-Hexane 110-54-3 < 5 665 68–70 1.5 1.375 n-Heptane 142-82-5 < 5 688 97–100 3.1 1.388 iso-Octane 540-84-1 < 5 696 98–101 2.9 1.392 (2,2,4-Trimethylpentane) n-Octane 111-65-9 < 5 708 124–127 8.0 1.398 n-Nonane* 111-84-2 < 5 -

Fatigue Craze Initiation in Polycarbonate: Study by Transmission Electron Microscopy
Fatigue craze initiation in polycarbonate: study by transmission electron microscopy Hristo A. Hristov, Albert F. Yee* and David W. Gidleyt Department of Materials Science and Engineering and tDepartment of Physics, University of Michigan, Ann Arbor, MI 48109, USA (Received 22 October 1993; revised 26 January 1994) Fatigue craze initiation in bulk, amorphous polycarbonate (PC) is investigated by means of transmission electron microscopy. The results agree very well with small angle X-ray scattering measurements of the same set of samples, and confirm that void generation is the main characteristic of the initiation process. The evolution of the initial void-like structures or 'protocrazes' with dimensions of ~ 50 nm into crazes with dimensions of several micrometres is presented in considerable detail. It is found that some similarities exist between crazes induced by cyclic fatigue at room temperature and crazes produced in monotonic loading at temperatures close to the glass transition. The similarities suggest that disentanglement of polymer chains plays an important role in the fatigue craze initiation process in bulk, amorphous PC near room temperature. (Keywords: fatigue; craze initiation; polycarbonate) INTRODUCTION describing fatigue craze initiation have not been The importance of crazing in the structural integrity of developed either (reviews of the existing models of craze polymeric materials under load was recognized more initiation under constant load can be found in references than 20 years ago, which prompted the beginning of 9 and 16). It seems that optical microscopy (conventional systematic investigations of structure of crazes in and interference optical microscopy) is the most polymers by, among other techniques, transmission frequently used method for studying the gross structure electron microscopy (TEM) 1-4. -

Material Safety Data Sheet (Msds/Sds) Was Prepared by Technical Personnel Based on Data That They Believe in Their Good Faith Judgment Is Accurate
Version: 1.0 Revision Date: 11-14-2014 SAFETY DATA SHEET 1. Identification Product identifier: PETROLEUM ETHER Other means of identification Product No.: 6128, H489, 9272, 4983, 4980, 9270, 4976, 9268, 4971, 9265 Recommended use and restriction on use Recommended use: Not available. Restrictions on use: Not known. Manufacturer/Importer/Supplier/Distributor Information Manufacturer Company Name: Avantor Performance Materials, Inc. Address: 3477 Corporate Parkway, Suite 200 Center Valley, PA 18034 Telephone: Customer Service: 855-282-6867 Fax: Contact Person: Environmental Health & Safety e-mail: [email protected] Emergency telephone number: 24 Hour Emergency: 908-859-2151 Chemtrec: 800-424-9300 2. Hazard(s) identification Hazard Classification Physical Hazards Flammable liquids Category 2 Health Hazards Germ Cell Mutagenicity Category 1B Carcinogenicity Category 1B Aspiration Hazard Category 1 Label Elements Hazard Symbol: Signal Word: Danger Hazard Statement: Highly flammable liquid and vapor. May be fatal if swallowed and enters airways. May cause genetic defects. May cause cancer. Precautionary SDS_US - SDS000001091 1/10 Version: 1.0 Revision Date: 11-14-2014 Statement Prevention: Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Use personal protective equipment as required. Keep away from heat/sparks/open flames/hot surfaces. No smoking. Response: IF exposed or concerned: Get medical advice/attention. IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Do NOT induce vomiting. Storage: Store locked up. Store in a well-ventilated place. Keep container tightly closed. Keep cool. Disposal: Dispose of contents/container to an appropriate treatment and disposal facility in accordance with applicable laws and regulations, and product characteristics at time of disposal. -

SUPERCRITICAL CARBON DIOXIDE EXTRACTION of SOUTHERN PINE and PONDEROSA PINE1 David C
SUPERCRITICAL CARBON DIOXIDE EXTRACTION OF SOUTHERN PINE AND PONDEROSA PINE1 David C. Ritter Scientist2 Department of Forest Products University of Minnesota St. Paul, MN 55108 and Alton G. Campbell Associate Professor Department of Forest Products University of Idaho Moscow, ID 83843 (Received November 1989) ABSTRACT Pine wood and bark were extracted with supercritical (SC) carbon dioxide under various experimental conditions. The extractive yields ranged from 20-60% relative to the total diethyl ether extractive content. The yields were dependent on temperature, pressure, particle size, and fluid to wood ratio. The addition of ethanol to bark particles prior to SC CO, extraction produced higher yields of extracts relative to extractions without the addition of ethanol. Gas chromatographic (GC) analysis of selected SC carbon dioxide extracts revealed that the concentration of resin acids, as well as the yield of pure abietic acid, increased with temperature at constant pressure. Fatty acids were more soluble in SC carbon dioxide relative to diethyl ether. The concentration of fatty acids in SC carbon dioxide extracts did not appear to follow definite trends. In addition, observation of the wood particles with scanning electron microscope (SEM) revealed that the supercritical extraction process did not appear to sig- nificantly alter the wood surface structure. Keywords: Southern pine, ponderosa pine, supercritical, carbon dioxide, extraction, scanning electron microscopy. INTRODUCTION Supercritical fluid (SCF) extraction is a rapidly developing technology that has great potential for separating and purifying high value products (Hoyer 1985; Williams 198 1; Schneider 1978). Carbon dioxide (CO,) is probably the most studied supercritical fluid since it is nonflammable, noncorrosive, nontoxic, and inexpensive (Brogle 1982). -
Safety Data Sheet: Petroleum Ether 40-60 °C
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH) Petroleum ether 40-60 °C ROTIPURAN® p.a., ACS, ISO article number: T173 date of compilation: 2021-05-25 Version: 1.1 en Revision: 2021-05-26 Replaces version of: 2021-05-25 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Identification of the substance Petroleum ether 40-60 °C ROTIPURAN® p.a., ACS, ISO Article number T173 Registration number (REACH) not relevant (mixture) 1.2 Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses: Laboratory chemical Laboratory and analytical use Uses advised against: Do not use for products which come into contact with foodstuffs. Do not use for private purposes (household). 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone:+49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data :Department Health, Safety and Environment sheet: e-mail (competent person): [email protected] 1.4 Emergency telephone number Name Street Postal Telephone Website code/city National Poisons Information Beaumont Road Dublin 9 01 809 2166 https:// Centre www.poisons.ie/ Beaumont Hospital SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 (CLP) Section Hazard class Cat- Hazard class and Hazard egory category statement 2.6 Flammable liquid 2 Flam. -
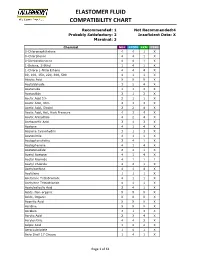
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air, -

The Growth of Cracks and Crazes in Polymers
THE GROWTH OF CRACKS AND CRAZES IN POLYMERS - A FRACTURE MECHANICS APPROACH GEORGE PHILIP MARSHALL MARCH W72 A thesis submitted for the degree of Doctor of Philosophy of the University of London and for the Diploma of Imperial College. Department of Mechanical Engineering Imperial College, . London S.W.7. ABSTRACT A study has been made of the use of fracture mechanics in describing crack and craze growth phenomena in plastics. The thesis which follows has been divided into three main parts. Part I has two component chapters. The first (Ch. 2)/ outlines the basis of the fracture mechanics concepts used in the analysis of results and the second chapter (Ch. 3) contains a literature survey of the state of knowledge on crazing and environmental stress cracking in plastics. Part II describes experimental work which has been undertaken to study crack growth phenomena in both air and liquid environments. Chapter 4 gives results obtained from slow crack propagation tests in PMMA in air and shows that there is a unique relationship between the fracture toughness measure Kc and the crack speed. Analysing results on a /crack speed basis is shown to ration- alise results from many different types of test and also accounts for the rate dependent material characteristics. This approach is extended in Chapter 5 to include the effects of crack propagation in polystyrene in air. For the first time, realistic values of fracture toughness have been obtained for the propaga- tion of a single crack/craze system. The Kc vs crack speed relationship is again found to provide a good basis against which results may be compared and dis- cussed. -
In Situ SEM Observation of Fracture Processes in Thin Film of Poly(Methyl Methacrylate) II
Polymer Journal, Vol.35, No. 1, pp 76—78 (2003) NOTES In Situ SEM Observation of Fracture Processes in Thin Film of Poly(methyl methacrylate) II. Stress Measurement Tetsuya NISHIURA Institute of Scientific and Industrial Research, Osaka University, 8–1 Mihogaoka, Ibaraki, Osaka 567–0047, Japan (Received August 6, 2002; Accepted October 17, 2002) KEY WORDS In situ Observation / Scanning Electron Microscope (SEM) / Craze / Fracture / Poly(methyl methacrylate) (PMMA) / Stress Observation / To investigate dynamic fracture processes of poly- mers on a microscopic scale, a fracture test of poly(methyl methacrylate) (PMMA) thin films was per- formed with scanning electron microscope (SEM) and fracture processes of the film were in situ observed.1 A crack is preceded by a craze. The mechanisms of prop- agation and fracture in the craze should be clarified. Figure 1. A load cell in tensile test machine. Test conditions affect nucleation of a craze, propagation of a craze and transformation to crack from the craze. Stress is an important factor in what yields a craze, and the relation between the stress and the craze behavior was studied. In bulk polymers, stress and stress inten- sity factors have been investigated.2, 3 In thin polymer film, craze stress is measured indirectly using an opti- cal density method.4 In previous work stress on the film subjected to tensile load was not detected.1 In this work, test equipment was improved to detect the tensile stress on the specimen. A small load cell was Figure 2. Calibration curve for output voltages vs. loads in a made from four strain gauges attached to a thin plate load cell. -

Fracture Toughness of Polypropylene-Based Particulate Composites
Materials 2009, 2, 2046-2094; doi:10.3390/ma2042046 OPEN ACCESS materials ISSN 1996-1944 www.mdpi.com/journal/materials Review Fracture Toughness of Polypropylene-Based Particulate Composites David Arencón and José Ignacio Velasco * Centre Català del Plàstic, Universitat Politècnica de Catalunya, Edifici Vapor Universitari, Colom 114, 08222, Terrassa, Spain; E-Mail: [email protected] (D.A.) * Author to whom correspondence should be addressed; E-Mail: [email protected] (J.I.V.); Tel.: +34 937 837 022; Fax: +34 937 841 827. Received: 27 October 2009; in revised form: 24 November 2009 / Accepted: 27 November 2009 / Published: 30 November 2009 Abstract: The fracture behaviour of polymers is strongly affected by the addition of rigid particles. Several features of the particles have a decisive influence on the values of the fracture toughness: shape and size, chemical nature, surface nature, concentration by volume, and orientation. Among those of thermoplastic matrix, polypropylene (PP) composites are the most industrially employed for many different application fields. Here, a review on the fracture behaviour of PP-based particulate composites is carried out, considering the basic topics and experimental techniques of Fracture Mechanics, the mechanisms of deformation and fracture, and values of fracture toughness for different PP composites prepared with different particle scale size, either micrometric or nanometric. Keywords: polypropylene; composites; fracture toughness 1. Introduction Particulate filled polymers are used in very large quantities in all kinds of applications and despite the overwhelming interest in advanced composite materials, considerable research and development is done on particulate filled polymers even today. Fillers increase stiffness and heat deflection temperatures, decrease shrinkage and improve the appearance of composites [1–3].