Apoptosis of Tumor-Infiltrating T Lymphocytes: a New Immune
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accumulation of P53 and Reductions in XIAP Abundance Promote the Apoptosis of Prostate Cancer Cells
Research Article Accumulation of p53 and Reductions in XIAP Abundance Promote the Apoptosis of Prostate Cancer Cells Subhra Mohapatra, Baoky Chu, Xiuhua Zhao, and W.J. Pledger Department of Interdisciplinary Oncology, H. Lee Moffitt Cancer Center and Research Institute and the University of South Florida Medical Center, Tampa, Florida Abstract activates caspase-9. Most drugs signal apoptosis through the Toward the goal of developing effective treatments for mitochondrial pathway. prostate cancers, we examined the effects of cyclin-dependent Proteins that modulate caspase activity and thus determine kinase inhibitors on the survival of prostate cancer cells. We whether cells live or die include the inhibitor of apoptosis proteins show that roscovitine, R-roscovitine, and CGP74514A (collec- (IAPs) and the Bcl-2 proteins. The IAP family includes cIAP-1, cIAP- tively referred to as CKIs) induce the apoptosis of LNCaP and 2, XIAP, and survivin (11). Of these proteins, XIAP is the most potent. LNCaP-Rf cells, both of which express wild-type p53. Apoptosis IAPs interact with and inhibit the activity of processed caspases; required caspase-9 and caspase-3 activity, and cytochrome c thus, they function as ‘‘brakes’’ that can impede the apoptotic accumulated in the cytosol of CKI-treated cells. Amounts of process once it begins. IAPs inactivate both initiator and effector caspases; caspase-9 and caspase-3 are IAP targets, whereas caspase- p53 increased substantially in CKI-treated cells, whereas amounts of the endogenous caspase inhibitor XIAP decreased. 8 is not (12). CKIs did not appreciably induce the apoptosis of LNCaP cells The Bcl-2 proteins are critical determinants of mitochondria- treated with pifithrin-A, which prevents p53 accumulation, or dependent caspase activation (13). -

Cytokine Nomenclature
RayBiotech, Inc. The protein array pioneer company Cytokine Nomenclature Cytokine Name Official Full Name Genbank Related Names Symbol 4-1BB TNFRSF Tumor necrosis factor NP_001552 CD137, ILA, 4-1BB ligand receptor 9 receptor superfamily .2. member 9 6Ckine CCL21 6-Cysteine Chemokine NM_002989 Small-inducible cytokine A21, Beta chemokine exodus-2, Secondary lymphoid-tissue chemokine, SLC, SCYA21 ACE ACE Angiotensin-converting NP_000780 CD143, DCP, DCP1 enzyme .1. NP_690043 .1. ACE-2 ACE2 Angiotensin-converting NP_068576 ACE-related carboxypeptidase, enzyme 2 .1 Angiotensin-converting enzyme homolog ACTH ACTH Adrenocorticotropic NP_000930 POMC, Pro-opiomelanocortin, hormone .1. Corticotropin-lipotropin, NPP, NP_001030 Melanotropin gamma, Gamma- 333.1 MSH, Potential peptide, Corticotropin, Melanotropin alpha, Alpha-MSH, Corticotropin-like intermediary peptide, CLIP, Lipotropin beta, Beta-LPH, Lipotropin gamma, Gamma-LPH, Melanotropin beta, Beta-MSH, Beta-endorphin, Met-enkephalin ACTHR ACTHR Adrenocorticotropic NP_000520 Melanocortin receptor 2, MC2-R hormone receptor .1 Activin A INHBA Activin A NM_002192 Activin beta-A chain, Erythroid differentiation protein, EDF, INHBA Activin B INHBB Activin B NM_002193 Inhibin beta B chain, Activin beta-B chain Activin C INHBC Activin C NM005538 Inhibin, beta C Activin RIA ACVR1 Activin receptor type-1 NM_001105 Activin receptor type I, ACTR-I, Serine/threonine-protein kinase receptor R1, SKR1, Activin receptor-like kinase 2, ALK-2, TGF-B superfamily receptor type I, TSR-I, ACVRLK2 Activin RIB ACVR1B -

Cancer Immunology, Immunotherapy Editor-In-Chief: H
Cancer Immunology, Immunotherapy Editor-in-Chief: H. Dong ▶ 94% of authors who answered a survey reported that they would definitely publish or probably publish in the journal again Since its inception in 1976, Cancer Immunology, Immunotherapy (CII) has reported significant advances in the field of tumor immunology. The journal serves as a forum for new concepts and advances in basic, translational, and clinical cancer immunology and immunotherapy. CII is keen to publish broad-ranging ideas and reviews, results which extend or challenge established paradigms, as well as negative studies which fail to reproduce experiments that support current paradigms, and papers that do succeed in reproducing others’ results in different contexts. Cll is especially interested in papers describing clinical trial designs and outcome regardless of whether they met their designated endpoints or not, and particularly those shedding light on immunological mechanisms. CII is affiliated with the Association for Cancer Immunotherapy (CIMT), Canadian Cancer 12 issues/year Immunotherapy Consortium (CCIC), The Japanese Association for Cancer Immunology (JACI), Network Italiano per la Bioterapia dei Tumori (NIBIT),and Sociedad Española de Inmunologia- Electronic access Grupo Española de InmunoTerapia (SEI-GEIT). ▶ link.springer.com CIMT: http://www.cimt.eu/home Subscription information CCIC: http://www.immunotherapycancer.ca/ ▶ springer.com/librarians JACI: http://www.jaci.jp/eng/index.html NIBIT: http://www.nibit.org/index.php SEI-GEIT: http://www.inmunologia.org/grupos/home.php? UpOm5=M&Upfqym5uom=GK CITIM: http://www.canceritim.org Impact Factor: 6.968 (2020), Journal Citation Reports® On the homepage of Cancer Immunology, Immunotherapy at springer.com you can ▶ Sign up for our Table of Contents Alerts ▶ Get to know the complete Editorial Board ▶ Find submission information. -

Prospects for NK Cell Therapy of Sarcoma
cancers Review Prospects for NK Cell Therapy of Sarcoma Mieszko Lachota 1 , Marianna Vincenti 2 , Magdalena Winiarska 3, Kjetil Boye 4 , Radosław Zago˙zd˙zon 5,* and Karl-Johan Malmberg 2,6,* 1 Department of Clinical Immunology, Doctoral School, Medical University of Warsaw, 02-006 Warsaw, Poland; [email protected] 2 Department of Cancer Immunology, Institute for Cancer Research, Oslo University Hospital, 0310 Oslo, Norway; [email protected] 3 Department of Immunology, Medical University of Warsaw, 02-097 Warsaw, Poland; [email protected] 4 Department of Oncology, Oslo University Hospital, 0310 Oslo, Norway; [email protected] 5 Department of Clinical Immunology, Medical University of Warsaw, 02-006 Warsaw, Poland 6 Center for Infectious Medicine, Department of Medicine Huddinge, Karolinska Institutet, Karolinska University Hospital, 141 86 Stockholm, Sweden * Correspondence: [email protected] (R.Z.); [email protected] (K.-J.M.) Received: 15 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Sarcomas are a group of aggressive tumors originating from mesenchymal tissues. Patients with advanced disease have poor prognosis due to the ineffectiveness of current treatment protocols. A subset of lymphocytes called natural killer (NK) cells is capable of effective surveillance and clearance of sarcomas, constituting a promising tool for immunotherapeutic treatment. However, sarcomas can cause impairment in NK cell function, associated with enhanced tumor growth and dissemination. In this review, we discuss the molecular mechanisms of sarcoma-mediated suppression of NK cells and their implications for the design of novel NK cell-based immunotherapies against sarcoma. -

Life, Death, and the Pursuit of Apoptosis
Downloaded from genesdev.cshlp.org on October 4, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Life, death, and the pursuit of apoptosis Eileen White Center for Advanced Biotechnology and Medicine and Department of Biological Sciences and the Cancer Institute of New Jersey, Rutgers University, Piscataway, New Jersey 08854 USA Apoptosis or programmed cell death is a genetically con- (Oltvai et al. 1993). Bcl-2 is expressed widely during em- trolled response for cells to commit suicide. The symp- bryogenesis (LeBrun et al. 1993; Novack and Korsmeyer toms of apoptosis are viability loss accompanied by cy- 1994), and its expression becomes more restricted in toplasmic boiling, chromatin condensation, and DNA adult tissues to those requiring long-term survival (stem fragmentation (Wyllie 1980). Pathologists and develop- cells, postmitotic neurons, and proliferating zones; mental biologists have cataloged the occurrences of ap- Hockenbery et al. 1991). optosis for many years based on these defined morpho- Apoptosis plays a major role in normal development, logical features, but what has propelled apoptosis into and it was expected that gain- or loss-of-function of a the forefront of basic research has been the identification death inhibitor such as Bcl-2 would have a phenotype in of genes that control cell death and the appreciation of transgenic mice. Bcl-2 was expected to regulate apopto- the role of apoptosis in development and disease. Regu- sis in lymphoid cells because of the role of Bcl-2 in hu- lation of cell death is essential for normal development man B cell lymphoma. Targeted Bcl-2 overexpression to and is an important defense against viral infection and the lymphoid system extends normal B cell survival the emergence of cancer. -

Engineered Marrow Macrophages for Cancer Therapy: Engorgement, Accumulation, Differentiation, and Acquired Immunity
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2017 Engineered Marrow Macrophages For Cancer Therapy: Engorgement, Accumulation, Differentiation, And Acquired Immunity Cory Alvey University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Pharmacology Commons Recommended Citation Alvey, Cory, "Engineered Marrow Macrophages For Cancer Therapy: Engorgement, Accumulation, Differentiation, And Acquired Immunity" (2017). Publicly Accessible Penn Dissertations. 2164. https://repository.upenn.edu/edissertations/2164 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/2164 For more information, please contact [email protected]. Engineered Marrow Macrophages For Cancer Therapy: Engorgement, Accumulation, Differentiation, And Acquired Immunity Abstract The ability of a macrophage to engulf and break down invading cells and other targets provides a first line of immune defense in nearly all tissues. This defining ability ot ‘phagos’ or devour can subsequently activate the entire immune system against foreign and diseased cells, and progress is now being made on a decades-old idea of directing macrophages to phagocytose specific targets such as cancer cells. Physical properties of cancer cells influence phagocytosis and relate via cytoskeleton forces to differentiation pathways in solid tumors. Here, SIRPα on macrophages from mouse and human marrow was inhibited to block recognition of CD47, a ‘marker of self.’ These macrophages were then systemically injected into mice with fluorescent human tumors. Within days, the tumors regressed, and fluorescence analyses showed that the more the SIRPα-inhibited macrophages engulfed, the more they accumulated within tumors. In vitro phagocytosis experiments on transwells revealed that macrophage migration through micropores was inhibited by eating. -

Expression of the Tumor Necrosis Factor Receptor-Associated Factors
Expression of the Tumor Necrosis Factor Receptor- Associated Factors (TRAFs) 1 and 2 is a Characteristic Feature of Hodgkin and Reed-Sternberg Cells Keith F. Izban, M.D., Melek Ergin, M.D, Robert L. Martinez, B.A., HT(ASCP), Serhan Alkan, M.D. Department of Pathology, Loyola University Medical Center, Maywood, Illinois the HD cell lines. Although KMH2 showed weak Tumor necrosis factor receptor–associated factors expression, the remaining HD cell lines also lacked (TRAFs) are a recently established group of proteins TRAF5 protein. These data demonstrate that consti- involved in the intracellular signal transduction of tutive expression of TRAF1 and TRAF2 is a charac- several members of the tumor necrosis factor recep- teristic feature of HRS cells from both patient and tor (TNFR) superfamily. Recently, specific members cell line specimens. Furthermore, with the excep- of the TRAF family have been implicated in promot- tion of TRAF1 expression, HRS cells from the three ing cell survival as well as activation of the tran- HD cell lines showed similar TRAF protein expres- scription factor NF- B. We investigated the consti- sion patterns. Overall, these findings demonstrate tutive expression of TRAF1 and TRAF2 in Hodgkin the expression of several TRAF proteins in HD. Sig- and Reed–Sternberg (HRS) cells from archived nificantly, the altered regulation of selective TRAF paraffin-embedded tissues obtained from 21 pa- proteins may reflect HRS cell response to stimula- tients diagnosed with classical Hodgkin’s disease tion from the microenvironment and potentially (HD). In a selective portion of cases, examination of contribute both to apoptosis resistance and cell HRS cells for Epstein-Barr virus (EBV)–encoded maintenance of HRS cells. -

Immunotherapy, Inflammation and Colorectal Cancer
cells Review Immunotherapy, Inflammation and Colorectal Cancer Charles Robert Lichtenstern 1,2, Rachael Katie Ngu 1,2, Shabnam Shalapour 1,2,* and Michael Karin 1,2,3 1 Department of Pharmacology, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA; karinoffi[email protected] 2 Laboratory of Gene Regulation and Signal Transduction, Department of Pharmacology, School of Medicine, University of California, San Diego, La Jolla, CA 92093, USA 3 Moores Cancer Center, University of California, San Diego, La Jolla, CA 92093, USA * Correspondence: [email protected] Received: 30 January 2020; Accepted: 3 March 2020; Published: 4 March 2020 Abstract: Colorectal cancer (CRC) is the third most common cancer type, and third highest in mortality rates among cancer-related deaths in the United States. Originating from intestinal epithelial cells in the colon and rectum, that are impacted by numerous factors including genetics, environment and chronic, lingering inflammation, CRC can be a problematic malignancy to treat when detected at advanced stages. Chemotherapeutic agents serve as the historical first line of defense in the treatment of metastatic CRC. In recent years, however, combinational treatment with targeted therapies, such as vascular endothelial growth factor, or epidermal growth factor receptor inhibitors, has proven to be quite effective in patients with specific CRC subtypes. While scientific and clinical advances have uncovered promising new treatment options, the five-year survival rate for metastatic CRC is still low at about 14%. Current research into the efficacy of immunotherapy, particularly immune checkpoint inhibitor therapy (ICI) in mismatch repair deficient and microsatellite instability high (dMMR–MSI-H) CRC tumors have shown promising results, but its use in other CRC subtypes has been either unsuccessful, or not extensively explored. -

Supplementary Table 2 Supplementary Table 1
Supplementary table 1 Rai/ Binet IGHV Cytogenetic Relative viability Fludarabine- Sex Outcome CD38 (%) IGHV gene ZAP70 (%) Treatment (s) Stage identity (%) abnormalities* increase refractory 1 M 0/A Progressive 14,90 IGHV3-64*05 99,65 28,20 Del17p 18.0% 62,58322819 FCR n.a. 2 F 0/A Progressive 78,77 IGHV3-48*03 100,00 51,90 Del17p 24.8% 77,88052021 FCR n.a. 3 M 0/A Progressive 29,81 IGHV4-b*01 100,00 9,10 Del17p 12.0% 36,48 Len, Chl n.a. 4 M 1/A Stable 97,04 IGHV3-21*01 97,22 18,11 Normal 85,4191657 n.a. n.a. Chl+O, PCR, 5 F 0/A Progressive 87,00 IGHV4-39*07 100,00 43,20 Del13q 68.3% 35,23314039 n.a. HDMP+R 6 M 0/A Progressive 1,81 IGHV3-43*01 100,00 20,90 Del13q 77.7% 57,52490626 Chl n.a. Chl, FR, R-CHOP, 7 M 0/A Progressive 97,80 IGHV1-3*01 100,00 9,80 Del17p 88.5% 48,57389901 n.a. HDMP+R 8 F 2/B Progressive 69,07 IGHV5-a*03 100,00 16,50 Del17p 77.2% 107,9656878 FCR, BA No R-CHOP, FCR, 9 M 1/A Progressive 2,13 IGHV3-23*01 97,22 29,80 Del11q 16.3% 134,5866919 Yes Flavopiridol, BA 10 M 2/A Progressive 0,36 IGHV3-30*02 92,01 0,38 Del13q 81.9% 78,91844953 Unknown n.a. 11 M 2/B Progressive 15,17 IGHV3-20*01 100,00 13,20 Del11q 95.3% 75,52880995 FCR, R-CHOP, BR No 12 M 0/A Stable 0,14 IGHV3-30*02 90,62 7,40 Del13q 13.0% 13,0939004 n.a. -

Beyond Tumor Necrosis Factor Receptor: TRADD Signaling in Toll-Like Receptors
Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors Nien-Jung Chen*†‡, Iok In Christine Chio*‡, Wen-Jye Lin*, Gordon Duncan*, Hien Chau*§, David Katz*¶, Huey-Lan Huang*ʈ, Kelly A. Pike*,**, Zhenyue Hao*, Yu-Wen Su*, Kazuo Yamamoto*, Rene´ e F. de Pooter††, Juan Carlos Zu´ n˜ iga-Pflu¨ cker††, Andrew Wakeham*, Wen-Chen Yeh*, and Tak W. Mak*‡‡ *The Campbell Family Institute for Breast Cancer Research, Ontario Cancer Institute, University Health Network and Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada M5G 2C1; †Institute of Microbiology and Immunology, School of Life Science, National Yang-Ming University, Taipei, Taiwan 112, Republic of China; and ††Department of Immunology, University of Toronto, Sunnybrook and Women’s College Health Sciences Centre, 2075 Bayview Avenue, Toronto, ON, Canada M4N 3M5 Contributed by Tak W. Mak, July 9, 2008 (sent for review June 24, 2008) Tumor necrosis factor receptor 1-associated death domain protein tory responses in vivo, but also that this molecule is involved in (TRADD) is the core adaptor recruited to TNF receptor 1 (TNFR1) germinal center (GC) formation, T cell costimulation, and TLR upon TNF␣ stimulation. In cells from TRADD-deficient mice, TNF␣- signaling. Our TRADD knockout mice represent a very useful mediated apoptosis and TNF␣-stimulated NF-B, JNK, and ERK tool for extending the ever-increasing list of TRADD functions activation are defective. TRADD is also important for germinal in vitro and in vivo. center formation, DR3-mediated costimulation of T cells, and TNF␣- mediated inflammatory responses in vivo. TRADD deficiency does Results not enhance IFN␥-induced signaling. -
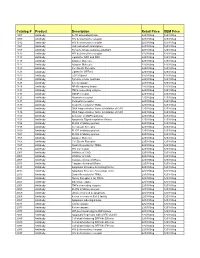
Comprehensive Product List
Catalog # Product Description Retail Price OEM Price 1007 Antibody IL-1R associated kinase 225/100ug 125/100ug 1009 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1012 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1021 Antibody JAK activated transcription 225/100ug 125/100ug 1107 Antibody Tyrosine kinase substrate p62DOK 225/100ug 125/100ug 1112 Antibody HIV & chemokine receptor 225/100ug 125/100ug 1113 Antibody Ligand for DR4 and DR5 225/100ug 125/100ug 1115 Antibody Adapter Molecule 225/100ug 125/100ug 1117 Antibody Adapter Molecule 225/100ug 125/100ug 1120 Antibody Cell Death Receptor 225/100ug 125/100ug 1121 Antibody Ligand for GFRa-2 225/100ug 125/100ug 1123 Antibody CCR3 ligand 225/100ug 125/100ug 1125 Antibody Tyrosine kinase substrate 225/100ug 125/100ug 1128 Antibody A new caspase 225/100ug 125/100ug 1129 Antibody NF-kB inducing kinase 225/100ug 125/100ug 1131 Antibody TNFa converting enzyme 225/100ug 125/100ug 1133 Antibody GDNF receptor 225/100ug 125/100ug 1135 Antibody Neurturin receptor 225/100ug 125/100ug 1137 Antibody Persephin receptor 225/100ug 125/100ug 1139 Antibody Death Receptor for TRAIL 225/100ug 125/100ug 1141 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1148 Antibody DNA fragmentation factor & Inhibitor of CAD 225/100ug 125/100ug 1150 Antibody Activator of MAPK pathway 225/100ug 125/100ug 1151 Antibody Apoptosis Signal-regulation Kinase 225/100ug 125/100ug 1156 Antibody FLICE inhibitory protein 225/100ug 125/100ug 1158 Antibody Cell Death Receptor 225/100ug 125/100ug 1159 -

Innate Immunity and Inflammation
ISBTc ‐ Primer on Tumor Immunology and Biological Therapy of Cancer InnateInnate ImmunityImmunity andand InflammationInflammation WillemWillem Overwijk,Overwijk, Ph.D.Ph.D. MDMD AndersonAnderson CancerCancer CenterCenter CenterCenter forfor CancerCancer ImmunologyImmunology ResearchResearch Houston,Houston, TXTX www.allthingsbeautiful.com InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications InnateInnate ImmunityImmunity andand InflammationInflammation • Definitions • Cells and Molecules • Innate Immunity and Inflammation in Cancer • Bad Inflammation • Good Inflammation • Therapeutic Implications • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. Adapted from Merriam‐Webster Medical Dictionary • Innate Immunity: Immunity that is naturally present and is not due to prior sensitization to an antigen; generally nonspecific. It is in contrast to acquired/adaptive immunity. • Inflammation: a local response to tissue injury – Rubor (redness) – Calor (heat) – Dolor (pain) – Tumor (swelling) Adapted from Merriam‐Webster Medical Dictionary ““InnateInnate ImmunityImmunity”” andand ““InflammationInflammation”” areare vaguevague termsterms •• SpecificSpecific cellcell typestypes andand moleculesmolecules orchestrateorchestrate specificspecific typestypes ofof inflammationinflammation