Student/Parent Handbook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![NCAA [R] Drug-Testing Program, 1999-2000](https://docslib.b-cdn.net/cover/2925/ncaa-r-drug-testing-program-1999-2000-42925.webp)
NCAA [R] Drug-Testing Program, 1999-2000
DOCUMENT RESUME ED 436 990 HE 032 608 AUTHOR Halpin, Ty, Ed. TITLE NCAA[R] Drug-Testing Program, 1999-2000. INSTITUTION National Collegiate Athletic Association, Indianapolis, IN. PUB DATE 1999-00-00 NOTE 15p. AVAILABLE FROM National Collegiate Athletic Association, P.O. Box 6222, Indianapolis, IN 46206-6222. Tel: 317-917-6222. PUB TYPE Legal/Legislative/Regulatory Materials (090) EDRS PRICE MF01/PC01 Plus Postage. DESCRIPTORS Athletes; *College Athletics; Drug Abuse; *Drug Use Testing; Higher Education; Illegal Drug Use IDENTIFIERS *National Collegiate Athletic Association ABSTRACT The drug testing program supports NCAA's goal to protect the health and safety of student-athletes competing for their institutions, while reaffirming the organization's commitment to fair and equitable competition. Proposal Nos. 30 and 52-54 provide a program for the NCAA's members to ensure that no one athlete has a chemically-induced advantage or is pressured to use chemical substances. The program involves urine collection on specific occasions and laboratory analyses for substances on a list of banned drugs including stimulants, anabolic agents such as steroids, diuretics, illegal drugs, or peptide hormones. Consent forms must be signed by student athletes if they wish to participate in NCAA programs. (JM) Reproductions supplied by EDRS are the best that can be made from the original document. 6' 3r; - RUG- ESTING ROGRAI4 1999-2000 , Pso (2 BEST COPY AVAILABLE U.S. DEPARTMENT OF EDUCATION Office of Educational Research and Improvement PERMISSION TO REPRODUCE AND EDUCATIONAL RESOURCES INFORMATION DISSEMINATE THIS MATERIAL HAS CENTER (ERIC) BEEN GRANTED BY his document has been reproduced as received from the person or organization originating it. -

Approval of Accounts Payable Bills
ÿÿ ÿÿ ÿ ÿÿ ÿÿ ÿÿ ÿÿÿÿ !"#$ÿÿ ÿÿ %&'ÿÿÿ($)01ÿÿ2#3ÿÿ ÿÿ 4567ÿ8!9!#ÿÿ@ABÿÿ@C@Dÿÿ ÿÿ E7ÿ 8FFGÿÿÿÿ8""!#ÿÿ210ÿÿ$ÿÿ ÿÿ E7H''7IP56QIÿÿ R#ÿÿ$ÿÿ"))ÿÿ# #ÿÿ# ÿÿÿÿÿÿ !"#$ÿÿFFGÿÿ# ÿÿF1)#ÿÿÿÿ""!#ÿÿF10ÿÿ0$ÿÿ$ÿÿ# ÿÿ )!#ÿÿÿÿSDBC@TBUVVWVÿÿ ÿÿ X5HY`&aIPÿÿ G1ÿÿ)# ÿÿÿÿ))0ÿÿÿÿ# ÿÿÿÿÿÿ !"#$ÿÿ$ÿÿ$9ÿÿ#ÿÿG$bÿÿ# ÿÿ" "3ÿÿ9$#ÿÿF$ÿÿ#ÿÿ# ÿÿ ÿÿ)#$9ÿÿ$ÿÿb $" ÿÿ# 1ÿÿb$ÿÿ0ÿÿF#ÿÿÿÿFFGÿÿÿc ÿÿ" "3ÿÿ9$#ÿÿ##" ÿÿ$$"#ÿÿ F1)#ÿÿ# #ÿÿ Gÿÿ0ÿÿG$bÿÿÿÿF"ÿÿ01ÿÿ# ÿÿ!$ÿÿdG$"ÿÿF#)#ÿÿ$ÿÿ# ÿÿ)ÿÿÿÿ FFÿÿ" "3ÿÿÿÿ!#)#ÿÿ"$9ÿÿ !ÿÿe8fgÿÿ"#$"ÿÿ#"#$ÿÿ ÿÿ 8ÿÿ!))1ÿÿÿÿ# ÿÿ""!#ÿÿF10ÿÿ0$ÿÿ$"!ÿÿÿÿFFGÿÿ$ÿÿÿÿbhÿÿ ÿÿ ip7HYÿÿ4567qrsÿÿ!1ÿÿ@UBÿÿ@C@DBÿÿ8!9!#ÿÿDCBÿÿ@C@DBÿÿtÿÿ8!9!#ÿÿ@uBÿÿ@C@Dÿÿ %aIPÿ%ÿÿ aIPÿÿ47rH&Qv6QIÿwÿ 'aI6ÿÿ DCÿÿ !"#$ÿSÿÿ TWVBT@DCTÿÿÿ @CÿxFÿÿ #$ÿÿÿÿy$#"ÿÿSÿ TDBuATUCÿÿÿ ACÿÿÿ 0#ÿÿdG$"ÿSÿÿ CCCÿÿ uCÿcÿÿ F##$ÿÿSÿ DDTBUWDUWÿÿÿ Cÿy!ÿÿ $"$Fÿÿ#$)#d"$ÿÿd"!$#1ÿÿSÿ CCCÿÿ WCÿÿÿ F$#ÿÿ2"#ÿÿSÿ AVBuATWÿÿÿ TCÿÿÿ 3$9ÿÿ ÿSÿÿ CCCÿÿ VCÿ ÿÿ $ÿÿ2G#$ÿÿtÿÿ$ÿÿd#1ÿSÿÿ CCCÿÿ Vÿÿÿ 03ÿÿ8!#$"ÿÿSÿ CCCÿÿ VWÿÿÿ ))!$#1ÿÿ29)ÿÿSÿ CCCÿÿ VVÿdÿÿ #!#ÿÿ8"#$G$#$ÿÿÿSÿÿ @VBuuA@Cÿÿÿ 65ÿSÿ DBC@TBUVVWVÿÿÿ ÿÿd#!#ÿÿ8"#$G$#$ÿÿF1)#ÿÿÿÿ$"!ÿÿb$# $ÿÿ# ÿÿ##" ÿÿ" "3ÿÿ9$#Bÿÿ0!#ÿÿF#ÿÿ #!#!ÿÿ#"#$ÿÿF#ÿÿ)ÿÿÿ$#$"#!ÿÿ#"#$ÿÿ ÿÿ ÿÿ ÿÿ ÿÿ ÿÿ ÿÿ ÿÿ ÿÿ ÿÿÿÿÿÿÿÿÿÿÿÿ !" ÿÿÿÿ #ÿÿ ! $ ÿÿ$ÿÿ ÿÿ %&'(')0ÿÿ12234536ÿÿ078552ÿÿ95@AÿÿBCDEÿÿ1F90ÿÿEGCDHIDPCQRÿÿSATU3V6ÿÿV8Aÿÿ678552ÿÿW5XT@ÿÿV5ÿÿ6YWU3VÿÿV5ÿÿV8Aÿÿ VTAX6YTATÿÿXÿÿ7ATV3`3A@ÿÿ75Saÿÿ5`ÿÿW5XT@ÿÿU34YVA6bÿÿST5SAT2aÿÿ63c4A@ÿÿWaÿÿV8Aÿÿ6A7TAVXTaÿÿX4@ÿÿSTA63@A4Vbÿÿ685d34cÿÿX22ÿÿ W3226ÿÿXSST5eA@ÿÿ`5TÿÿSXaUA4VÿÿWaÿÿV8AÿÿW5XT@ÿÿX4@ÿÿ72AXT2aÿÿ685d34cÿÿV5ÿÿd85UbÿÿX4@ÿÿ`5Tÿÿd8XVÿÿSYTS56AÿÿAX78ÿÿ SXaUA4Vÿÿ36ÿÿV5ÿÿWAÿÿUX@AÿÿWaÿÿV8AÿÿVTAX6YTATbÿÿX4@ÿÿV5ÿÿd8XVÿÿWY@cAVXTaÿÿ3VAUÿÿÿAX78ÿÿSXaUA4Vÿÿ68X22ÿÿWAÿÿ@AW3VA@fÿÿX4@ÿÿ -

Properties and Units in Clinical Pharmacology and Toxicology
Pure Appl. Chem., Vol. 72, No. 3, pp. 479–552, 2000. © 2000 IUPAC INTERNATIONAL FEDERATION OF CLINICAL CHEMISTRY AND LABORATORY MEDICINE SCIENTIFIC DIVISION COMMITTEE ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)# and INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY CHEMISTRY AND HUMAN HEALTH DIVISION CLINICAL CHEMISTRY SECTION COMMISSION ON NOMENCLATURE, PROPERTIES, AND UNITS (C-NPU)§ PROPERTIES AND UNITS IN THE CLINICAL LABORATORY SCIENCES PART XII. PROPERTIES AND UNITS IN CLINICAL PHARMACOLOGY AND TOXICOLOGY (Technical Report) (IFCC–IUPAC 1999) Prepared for publication by HENRIK OLESEN1, DAVID COWAN2, RAFAEL DE LA TORRE3 , IVAN BRUUNSHUUS1, MORTEN ROHDE1, and DESMOND KENNY4 1Office of Laboratory Informatics, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark; 2Drug Control Centre, London University, King’s College, London, UK; 3IMIM, Dr. Aiguader 80, Barcelona, Spain; 4Dept. of Clinical Biochemistry, Our Lady’s Hospital for Sick Children, Crumlin, Dublin 12, Ireland #§The combined Memberships of the Committee and the Commission (C-NPU) during the preparation of this report (1994–1996) were as follows: Chairman: H. Olesen (Denmark, 1989–1995); D. Kenny (Ireland, 1996); Members: X. Fuentes-Arderiu (Spain, 1991–1997); J. G. Hill (Canada, 1987–1997); D. Kenny (Ireland, 1994–1997); H. Olesen (Denmark, 1985–1995); P. L. Storring (UK, 1989–1995); P. Soares de Araujo (Brazil, 1994–1997); R. Dybkær (Denmark, 1996–1997); C. McDonald (USA, 1996–1997). Please forward comments to: H. Olesen, Office of Laboratory Informatics 76-6-1, Copenhagen University Hospital (Rigshospitalet), 9 Blegdamsvej, DK-2100 Copenhagen, Denmark. E-mail: [email protected] Republication or reproduction of this report or its storage and/or dissemination by electronic means is permitted without the need for formal IUPAC permission on condition that an acknowledgment, with full reference to the source, along with use of the copyright symbol ©, the name IUPAC, and the year of publication, are prominently visible. -

Prohibited Substances List
Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). Neither the List nor the EADCM Regulations are in current usage. Both come into effect on 1 January 2010. The current list of FEI prohibited substances remains in effect until 31 December 2009 and can be found at Annex II Vet Regs (11th edition) Changes in this List : Shaded row means that either removed or allowed at certain limits only SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin NSAID 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/anti-pyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic/anti-pyretic 8 Acetophenazine Antipsychotic 9 Acetylmorphine Narcotic 10 Adinazolam Anxiolytic 11 Adiphenine Anti-spasmodic 12 Adrafinil Stimulant 13 Adrenaline Stimulant 14 Adrenochrome Haemostatic 15 Alclofenac NSAID 16 Alcuronium Muscle relaxant 17 Aldosterone Hormone 18 Alfentanil Narcotic 19 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 20 Almotriptan 5 HT agonist (anti-migraine) 21 Alphadolone acetate Neurosteriod 22 Alphaprodine Opiod analgesic 23 Alpidem Anxiolytic 24 Alprazolam Anxiolytic 25 Alprenolol Beta blocker 26 Althesin IV anaesthetic 27 Althiazide Diuretic 28 Altrenogest (in males and gelidngs) Oestrus suppression 29 Alverine Antispasmodic 30 Amantadine Dopaminergic 31 Ambenonium Cholinesterase inhibition 32 Ambucetamide Antispasmodic 33 Amethocaine Local anaesthetic 34 Amfepramone Stimulant 35 Amfetaminil Stimulant 36 Amidephrine Vasoconstrictor 37 Amiloride Diuretic 1 Prohibited Substances List This is the Equine Prohibited Substances List that was voted in at the FEI General Assembly in November 2009 alongside the new Equine Anti-Doping and Controlled Medication Regulations(EADCMR). -

The M Street Journal Radio's Journal of Record ' EW YORK NASHVILLE CAPSTAR ACROSS AFRICA
The M Street Journal Radio's Journal of Record ' EW YORK NASHVILLE CAPSTAR ACROSS AFRICA. Capstar Broadcasting Partners will spend $60 million for twenty stations in four separate transactions covering five markets. Terms of the individual deals weren't disclosed. Two of the deals involve Point Communications, which is the managing partner of six stations in Madison, WI and owns five in the Roanoke - Lynchburg area, owned through a subsidiary. In Madison, the stations are standards WTSO; CHR WZEE; news -talk WIBA; rock WIBA -FM; new rock WMAD -FM, Sun Prairie, WI; and soft AC WMLI, Sauk City, WI. In Roanoke - Lynchburg -- oldies simulcast WLDJ, Appomattox and WRDJ, Roanoke; urban oldies WJJS, Lynchburg; and dance combo WJJS -FM, Vinton, and WJJX, Lynchburg. The third deal gives Capstar three stations in the Yuma, AZ market, including oldies KBLU, country KTTI, and classic rocker KYJT, from Commonwealth Broadcasting of Arizona, LLC. Finally, COMCO Broadcasting's Alaska properties, which include children's KYAK, CHR KGOT, and AC KYMG, all Anchorage; and news -talk KIAK, country KIAK -FM, and AC KAKQ -FM, all Fairbanks. WE DON'T NEED NO STINKIN' LICENSE . It's spent almost ten weeks on the air without a license, but the new religious -programmed station on 105.3 MHz in the Hartford, CT area, is being investigated by the Commission's New England Field Office. According to the Hartford Courant, Mark Blake is operating the station from studios in Bloomfield, CT, and says that he "stands behind" the station's operation. Although there have been no interference complaints filed, other stations in the area are claiming they are losing advertising dollars to the pirate. -
U. S. Radio Stations As of June 30, 1922 the Following List of U. S. Radio
U. S. Radio Stations as of June 30, 1922 The following list of U. S. radio stations was taken from the official Department of Commerce publication of June, 1922. Stations generally operated on 360 meters (833 kHz) at this time. Thanks to Barry Mishkind for supplying the original document. Call City State Licensee KDKA East Pittsburgh PA Westinghouse Electric & Manufacturing Co. KDN San Francisco CA Leo J. Meyberg Co. KDPT San Diego CA Southern Electrical Co. KDYL Salt Lake City UT Telegram Publishing Co. KDYM San Diego CA Savoy Theater KDYN Redwood City CA Great Western Radio Corp. KDYO San Diego CA Carlson & Simpson KDYQ Portland OR Oregon Institute of Technology KDYR Pasadena CA Pasadena Star-News Publishing Co. KDYS Great Falls MT The Tribune KDYU Klamath Falls OR Herald Publishing Co. KDYV Salt Lake City UT Cope & Cornwell Co. KDYW Phoenix AZ Smith Hughes & Co. KDYX Honolulu HI Star Bulletin KDYY Denver CO Rocky Mountain Radio Corp. KDZA Tucson AZ Arizona Daily Star KDZB Bakersfield CA Frank E. Siefert KDZD Los Angeles CA W. R. Mitchell KDZE Seattle WA The Rhodes Co. KDZF Los Angeles CA Automobile Club of Southern California KDZG San Francisco CA Cyrus Peirce & Co. KDZH Fresno CA Fresno Evening Herald KDZI Wenatchee WA Electric Supply Co. KDZJ Eugene OR Excelsior Radio Co. KDZK Reno NV Nevada Machinery & Electric Co. KDZL Ogden UT Rocky Mountain Radio Corp. KDZM Centralia WA E. A. Hollingworth KDZP Los Angeles CA Newbery Electric Corp. KDZQ Denver CO Motor Generator Co. KDZR Bellingham WA Bellingham Publishing Co. KDZW San Francisco CA Claude W. -

Correspondence
SEPT. 26, 1959 TO-DAY'S DRUGS MEDICAL JOURNAL 577 convulsions. The electroencephalogram changes can be reversed and the convulsions stopped by the intravenous administration of sodium thiopentone. Correspondence Although at irst it was thought that bemegride had a specific action in antagonizing the depressant effects of Because of heavy pressure on our space, correspondents are barbiturates on the central nervous system, this view is now asked to keep their letters short. no longer held. Bemegride is looked upon as a non- specific stimulant of the central nervous system and beneficial in treating cases of poisoning by various other Isaac Wolfson Foundation's Fund narcotics (other than barbiturates), such as primidone and glutethimide, as well as barbiturates. `SIR,-I would like to thank you for your leading Therapeutic uses of bemegride are as follows: article on the subject of the recent magnificent grant to (1) In the treatment of acute barbiturate poisoning. Here, in the Royal College of Physicians (Journal, September 12, addition to the usual general method of dealing with a comatose p. 416) and for giving space to a description of the patient, a 5% glucose saline intravenous infusion is given slowly, important activities of the Isaac Wolfson Foundation and 10 ml. of 0.5% solution of bemegride (50 mg.) is injected (p. 427). This grant is indeed a historic gift which my into the tubing leading to the intravenous cannula every three and Medicine are not to to five minutes. Some clinicians'precede each injection with 1 ml. College British likely forget. of a 1.5% solution of amiphenazole (15 mg.). -
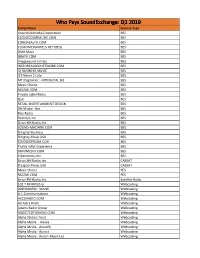
Licensee Count Q1 2019.Xlsx
Who Pays SoundExchange: Q1 2019 Entity Name License Type Aura Multimedia Corporation BES CLOUDCOVERMUSIC.COM BES COROHEALTH.COM BES CUSTOMCHANNELS.NET (BES) BES DMX Music BES GRAYV.COM BES Imagesound Limited BES INSTOREAUDIONETWORK.COM BES IO BUSINESS MUSIC BES It'S Never 2 Late BES MTI Digital Inc - MTIDIGITAL.BIZ BES Music Choice BES MUZAK.COM BES Private Label Radio BES Qsic BES RETAIL ENTERTAINMENT DESIGN BES Rfc Media - Bes BES Rise Radio BES Rockbot, Inc. BES Sirius XM Radio, Inc BES SOUND-MACHINE.COM BES Stingray Business BES Stingray Music USA BES STUDIOSTREAM.COM BES Thales Inflyt Experience BES UMIXMEDIA.COM BES Vibenomics, Inc. BES Sirius XM Radio, Inc CABSAT Stingray Music USA CABSAT Music Choice PES MUZAK.COM PES Sirius XM Radio, Inc Satellite Radio 102.7 FM KPGZ-lp Webcasting 999HANKFM - WANK Webcasting A-1 Communications Webcasting ACCURADIO.COM Webcasting Ad Astra Radio Webcasting Adams Radio Group Webcasting ADDICTEDTORADIO.COM Webcasting Aloha Station Trust Webcasting Alpha Media - Alaska Webcasting Alpha Media - Amarillo Webcasting Alpha Media - Aurora Webcasting Alpha Media - Austin-Albert Lea Webcasting Alpha Media - Bakersfield Webcasting Alpha Media - Biloxi - Gulfport, MS Webcasting Alpha Media - Brookings Webcasting Alpha Media - Cameron - Bethany Webcasting Alpha Media - Canton Webcasting Alpha Media - Columbia, SC Webcasting Alpha Media - Columbus Webcasting Alpha Media - Dayton, Oh Webcasting Alpha Media - East Texas Webcasting Alpha Media - Fairfield Webcasting Alpha Media - Far East Bay Webcasting Alpha Media -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Appendix B Banned Drugs
APPENDIX B BANNED DRUGS (This list complies with 2004-2005 NCAA Bylaw 31.2.3.1 Banned Drugs) A. Stimulants: amiphenazole amphetamine bemigride benzphetamine bromantan caffeine1 (guarana) chlorphentermine cocaine cropropamide crothetamide diethylpropion dimethylamphetamine doxapram ephedrine (ephedra, ma huang) ethamivan ethylamphetamine fencamfamine meclofenoxate methamphetamine methylene-dioxymethamphetamine [MDMA (ecstasy)] methylphenidate nikethamide pemoline pentetrazol phendimetrazine phenmetrazine phentermine phenylephrine phenylpropanolamine (ppa) picrotoxine pipradol prolintane strychnine synephrine (citrus aurantium, zhi shi, bitter orange) and related compounds Approved by Cabinet:1/31/05 Adopted by Board of Trustees:3/18/05 Page 12 of 15 B. Anabolic Agents: polythiazide anabolic steroids quinethazone androstenediol spironolactone androstenedione triamterene boldenone trichlormethiazide clostebol and related compounds dehydrochlormethyltestosterone dehydroepiandrosterone (DHEA) dihydrotestosterone (DHT) D. Street Drugs: dromostanolone heroin fluoxymesterone marijuana3 gestrinone THC mesterolone (tetrahydrocannabinol3 methandienone methenolone methyltestosterone nandrolone norandrostenediol norandrostenedione norethandrolone oxandrolone oxymesterone oxymetholone stanozolol testosterone2 tetrahydrogestrinone (THG) trenbolone and related compounds other anabolic agents clenbuterol C. Diuretics: acetazolamide bendroflumethiazide benzthiazide bumetanide chlorothiazide chlorthalidone ethacrynic acid flumethiazide furosemide hydrochlorothiazide -

Pharmacology of Stimulants Prohibited by the World Anti-Doping Agency (WADA)
British Journal of Pharmacology (2008) 154, 606–622 & 2008 Nature Publishing Group All rights reserved 0007– 1188/08 $30.00 www.brjpharmacol.org REVIEW Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA) JR Docherty Department of Physiology, Royal College of Surgeons in Ireland, Dublin, Ireland This review examines the pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA). Stimulants that increase alertness/reduce fatigue or activate the cardiovascular system can include drugs like ephedrine available in many over- the-counter medicines. Others such as amphetamines, cocaine and hallucinogenic drugs, available on prescription or illegally, can modify mood. A total of 62 stimulants (61 chemical entities) are listed in the WADA List, prohibited in competition. Athletes may have stimulants in their body for one of three main reasons: inadvertent consumption in a propriety medicine; deliberate consumption for misuse as a recreational drug and deliberate consumption to enhance performance. The majority of stimulants on the list act on the monoaminergic systems: adrenergic (sympathetic, transmitter noradrenaline), dopaminergic (transmitter dopamine) and serotonergic (transmitter serotonin, 5-HT). Sympathomimetic describes agents, which mimic sympathetic responses, and dopaminomimetic and serotoninomimetic can be used to describe actions on the dopamine and serotonin systems. However, many agents act to mimic more than one of these monoamines, so that a collective term of monoaminomimetic may be useful. Monoaminomimietic actions of stimulants can include blockade of re-uptake of neurotransmitter, indirect release of neurotransmitter, direct activation of monoaminergic receptors. Many of the stimulants are amphetamines or amphetamine derivatives, including agents with abuse potential as recreational drugs. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic