The Platinum Metals in the Periodic System a COMPARATIVE STUDY of the TRANSITION METALS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Osmium(II) Polypyridyl Complexes Wesley R. Browne a Thesis Pres
Probing ground and excited state properties of Ruthenium(II) and Osmium(II) polypyridyl complexes Wesley R. Browne A Thesis presented to Dublin City University for the degree of Doctor of Philosophy. 2002 Probing ground and excited state properties of Ruthenium(II) and Osmium(II) polypyridyl complexes Isotope, pH, solvent and temperature effects. by Wesley R. Browne, BSc.(Hons), AMRSC A Thesis presented to Dublin City University for the degree of Doctor of Philosophy. Supervisor: Professor Johannes G. Vos School of Chemical Sciences Dublin City University August 2002 Dedicated to Matthew & to the memory of Ross and Mick “Caste a cold eye on life, on death. Horseman pass by” Epitaph fo r William Butler Yeats REFERENCE I hereby certify that this material, which I now submit for assessment on the programme of study leading to the award of Doctor of Philosophy by research and thesis, is entirely my own work and has not been taken from work of others, save and to the extent that such work has been cited within the text of my work. I D. No. 95478965 Date:_______ ( ( / Û / iv Abstract The area of ruthenium(H) and osmium(H) polypyridyl chemistry has been the subject of intense investigation over the last half century. In chapter 1, topics relevant to the studies presented in this thesis are introduced. These areas include the basic principles behind the ground and excited state properties of Ru(II) and Os(II) polypyridyl complexes, complexes incorporating the 1,2,4-triazole moiety and the application of deuteriation to inorganic photophysics. Chapter 2 details experimental and basic synthetic procedures employed in the studies presented in later chapters. -

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -
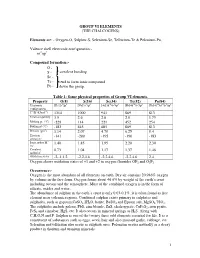
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Iron-Catalyzed Reactions and X-Ray Absorption Spectroscopic Studies
Arnar Guðmundsson Iron-Catalyzed Reactions and X-Ray Absorption Spectroscopic Studies of Palladium- and Iron-Catalyzed Reactions and X-Ray Absorption Spectroscopic Studies of Palladium- and Ruthenium-Catalyzed Reactions and Ruthenium-Catalyzed Studies of Palladium- Absorption Spectroscopic Reactions and X-Ray Iron-Catalyzed Ruthenium-Catalyzed Reactions Arnar Guðmundsson Arnar Guðmundsson was born in Reykjavík, Iceland. After finishing his bachelor studies in biochemistry at the University of Iceland, he moved to Sweden to pursue his masters and doctoral studies under the guidance of Prof. Jan-Erling Bäckvall. ISBN 978-91-7911-264-6 Department of Organic Chemistry Doctoral Thesis in Organic Chemistry at Stockholm University, Sweden 2020 Iron-Catalyzed Reactions and X-Ray Absorption Spectroscopic Studies of Palladium- and Ruthenium-Catalyzed Reactions Arnar Guðmundsson Academic dissertation for the Degree of Doctor of Philosophy in Organic Chemistry at Stockholm University to be publicly defended on Friday 22 January 2021 at 10.00 in Magnélisalen, Kemiska övningslaboratoriet, Svante Arrhenius väg 16 B. Abstract The focus of this thesis is twofold: The first is on the application of iron catalysis for organic transformations. The second is on the use of in situ X-ray absorption spectroscopy (XAS) to investigate the mechanisms of a heterogeneous palladium- catalyzed reaction and a homogeneous ruthenium-catalyzed reaction. In chapters two, three and four, the use of iron catalyst VI, or its analog X, is described for (I) the DKR of sec-alcohols to produce enantiomerically pure acetates; (II) the cycloisomerization of α-allenols and α-allenic sulfonamides, giving 2,3-dihydrofuran or 2,3-dihydropyrrole products, respectively, with excellent diastereoselectivity; and (III) the aerobic biomimetic oxidation of primary- and secondary alcohols to their respective aldehydes or ketones. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure. -

In-System'' Fission-Events: an Insight Into Puzzles of Exoplanets and Stars?
universe Review “In-System” Fission-Events: An Insight into Puzzles of Exoplanets and Stars? Elizabeth P. Tito 1,* and Vadim I. Pavlov 2,* 1 Scientific Advisory Group, Pasadena, CA 91125, USA 2 Faculté des Sciences et Technologies, Université de Lille, F-59000 Lille, France * Correspondence: [email protected] (E.P.T.); [email protected] (V.I.P.) Abstract: In expansion of our recent proposal that the solar system’s evolution occurred in two stages—during the first stage, the gaseous giants formed (via disk instability), and, during the second stage (caused by an encounter with a particular stellar-object leading to “in-system” fission- driven nucleogenesis), the terrestrial planets formed (via accretion)—we emphasize here that the mechanism of formation of such stellar-objects is generally universal and therefore encounters of such objects with stellar-systems may have occurred elsewhere across galaxies. If so, their aftereffects may perhaps be observed as puzzling features in the spectra of individual stars (such as idiosyncratic chemical enrichments) and/or in the structures of exoplanetary systems (such as unusually high planet densities or short orbital periods). This paper reviews and reinterprets astronomical data within the “fission-events framework”. Classification of stellar systems as “pristine” or “impacted” is offered. Keywords: exoplanets; stellar chemical compositions; nuclear fission; origin and evolution Citation: Tito, E.P.; Pavlov, V.I. “In-System” Fission-Events: An 1. Introduction Insight into Puzzles of Exoplanets As facilities and techniques for astronomical observations and analyses continue to and Stars?. Universe 2021, 7, 118. expand and gain in resolution power, their results provide increasingly detailed information https://doi.org/10.3390/universe about stellar systems, in particular, about the chemical compositions of stellar atmospheres 7050118 and structures of exoplanets. -

Physical and Chemical Properties of Platinum Group Metals 2 Chapter 2 | Physical and Chemical Properties of Platinum Group Metals Contents
PHYSICAL AND CHEMICAL PROPERTIES OF PLATINUM GROUP METALS 2 CHAPTER 2 | PHYSICAL AND CHEMICAL PROPERTIES OF PLATINUM GROUP METALS CONTENTS 2.1 OVERVIEW OF PGMS 04 2.2 METALLIC PGMS 05 2.3 COMPOUNDS OF PLATINUM GROUP METALS 06 SIMPLE COMPOUNDS 06 COMPLEX COMPOUNDS 07 REFERENCES 13 2 CHAPTER 2 | PHYSICAL AND CHEMICAL PROPERTIES OF PLATINUM GROUP METALS SUMMARY • Six elements of Groups 8, 9, and 10 in the periodic table constitute the platinum group metals (PGMs): platinum (Pt), palladium (Pd), rhodium (Rh), ruthenium (Ru), iridium (Ir), and osmium (Os). • The physical and mechanical properties of the PGMs and their compounds indicate a wide range of properties with widely varying densities and solubilities (see Table 2-1). • Metallic forms of PGMs are generally considered to be ‘inert’, i.e., not chemically reactive. However, this is dependent in part on dimensional characteristics. Thus, while massive metal forms have low chemical reactivity, fi nely-divided metal powders with high surface area show greater reactivity. • Simple binary compounds exist for each of the PGMs. They also form a vast array of complex coordination compounds in which the central metal atom is bound to a variety of ligands by coordinate bonding, including halides, sulphur, amines, and other atoms and groups. • This unique coordination chemistry has made PGM compounds of great industrial value, but also can have implications for the health of workers exposed to certain of these compounds due to the linkages with biological behaviour and toxicity (see Chapter 6). • The complex halogenated platinum compounds (CHPS) are among those which are industrially and toxicologically important.