Antibody-Free Colorimetric Detection of Chlorothalonil in Cucumber Via the Inhibition of an Cite This: RSC Adv.,2019,9, 9893 Enzyme-Triggered Reaction†
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
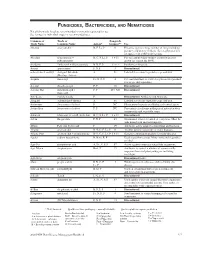
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Concentration, Reduction Efficancy and Degradation of Chlorothalonil and Lambda Cyhalothrin Pesticides in Vegetables Sold in a Nairobi City Market
UNIVERSITY OF NAIROBI CONCENTRATION, REDUCTION EFFICANCY AND DEGRADATION OF CHLOROTHALONIL AND LAMBDA CYHALOTHRIN PESTICIDES IN VEGETABLES SOLD IN A NAIROBI CITY MARKET BY JAMES MUNGAI I56/80825/2015 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR AWARD OF THE DEGREE OF MASTER OF SCIENCE IN ANALYTICAL CHEMISTRY OF THE UNIVERSITY OF NAIROBI AUGUST 2020 i DECLARATION I declare that this research work is my original work and has not been submitted to any institution for any academic award. Where other people’s works have been cited, this has properly been acknowledged and referenced in accordance with the University of Nairobi’s requirements. SIGN……………………………… DATE……………….............. JAMES MUNGAI I56/80825/2015 This thesis is submitted for examination with our approval as the university supervisors. Signature ………………………….. Date …………………… DR. JOYCE G. N. KITHURE DEPARTMENT OF CHEMISTRY UNIVERSITY OF NAIROBI Signature ………………………….. Date …………………… DR. VINCENT O. MADADI DEPARTMENT OF CHEMISTRY UNIVERSITY OF NAIROBI Signature ………………………….. Date …………………… DR. DEBORAH A. ABONG’O DEPARTMENT OF CHEMISTRY UNIVERSITY OF NAIROBI ii DEDICATION This research work is dedicated to my inspiration and mentor the late Professor G.N. Kamau for his selfless support, guidance and professional advice during early stages of this study. iii ACKNOWLEDGEMENT In the course of my research work, I have enjoyed inspiration, love, support, guidance and co-operation. I am very grateful to God for the opportunity He has given to me to undertake this study. I give special thanks posthumously to the late Prof. G. N. Kamau for his inspiration, professional advice and valuable guidance. I am very grateful to Dr. Joyce G. N. -

SDS # : FO001139-1-A Revision Date: 2016-05-26 Format: NA Version 1.02
SAFETY DATA SHEET Fame™ +C SC Fungicide SDS # : FO001139-1-A Revision date: 2016-05-26 Format: NA Version 1.02 1. PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Name Fame™ +C SC Fungicide Other means of identification Product Code(s) FO001139-1-A Synonyms FLUOXASTROBIN: (E)-{ 2-[6-(2-chlorophenoxy)-5-fluoropyrimidin-4-yloxy]phenyl} (5,6-dihydro-1,4,2-dioxazin-3-yl)methanone O-methyloxime (IUPAC name); (1E)-[2-[[6-(2-chlorophenoxy)-5-fluoro-4-pyrimidinyl]oxy]phenyl](5,6-dihydro-1,4,2-dioxazin- 3-yl)methanone O-methyloxime (CAS name) CHLOROTHALONIL: tetrachloroisophthalonitrile (IUPAC name); 2,4,5,6-tetrachloro-1,3-benzenedicarbonitrile (CAS name) Active Ingredient(s) Fluoxastrobin, Chlorothalonil Chemical Family Strobiluron fungicide, Broad spectrum, nonsystemic fungicide Recommended use of the chemical and restrictions on use Recommended Use: Fungicide Restrictions on Use: Use as recommended by the label Manufacturer Address FMC Corporation 2929 Walnut Street Philadelphia, PA 19104 (215) 299-6000 (General Information) [email protected] (E-Mail General Information) Emergency telephone number For leak, fire, spill or accident emergencies, call: 1 800 / 424 9300 (CHEMTREC - U.S.A.) 1 703 / 527 3887 (CHEMTREC - Collect - All Other Countries) Medical Emergencies: 1 800 / 331-3148 (PROSAR - U.S.A. & Canada) 1 651 / 632-6793 (PROSAR - All Other Countries - Collect) 2. HAZARDS IDENTIFICATION Classification OSHA Regulatory Status This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute -

Workshop Report on the Application of 2,3,7,8-TCDD Toxicity Equivalence Factors to Fish and Wildlife
EPA/630/R-01/002 August 2001 www.epa.gov/ncea/raf Workshop Report on the Application of 2,3,7,8-TCDD Toxicity Equivalence Factors to Fish and Wildlife Risk Assessment Forum U.S. Environmental Protection Agency Washington, DC DISCLAIMER This document has been reviewed in accordance with U.S. Environmental Protection Agency policy and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. ii CONTENTS CONTRIBUTORS TO WORKSHOP DESIGN AND REPORT ........................ iv 1. INTRODUCTION ..........................................................1 2. OBJECTIVES AND DESIGN OF THE EPA/DOI WORKSHOP ......................4 3. WORKSHOP FINDINGS.....................................................6 4. CONCLUSIONS OF THE EPA/DOI WORKSHOP PLANNING GROUP ..............11 5. REFERENCES............................................................18 APPENDICES: A. Workshop Case Study: A Preliminary Problem Formulation for a Retrospective Ecological Risk Assessment Scenario.................................................... A-1 B. Workshop Case Study: A Preliminary Problem Formulation for a Prospective Ecological Risk Assessment Scenario.....................................................B-1 C. Report from the Workshop on the Application of 2,3,7,8-TCDD Toxicity Equivalence Factors to Fish and Wildlife.................................................... C-i I. Introduction ........................................................C-1 II. Opening Presentations...............................................C-2 -

Particularly Hazardous Substances
Particularly Hazardous Substances In its Laboratory Standard, OSHA requires the establishment of additional protections for persons working with "Particularly Hazardous Substances" (PHS). OSHA defines these materials as "select" carcinogens, reproductive toxins and acutely toxic materials. Should you wish to add: explosive, violently reactive, pyrophoric and water-reactve materials to this category, the information is included. Carbon nanotubes have also been added due to their suspected carcinogenic properties. This table is designed to assist the laboratory in the identification of PHS, although it is not definitively conclusive or entirely comprehensive. *Notes on the proper use of this table appear on page 12. 1 6 5 2 3 4 Substance CAS National Toxicity National Program Carcinogen Toxin Acute Regulated OSHA Carcinogen Group IARC Carcinogen Toxin Reproductive Violently Reactive/ Explosive/Peroxide Forming/Pyrophoric A-a-C(2-Amino-9H-pyrido[2,3,b]indole) 2648-68-5 2B Acetal 105-57-7 yes Acetaldehyde 75-07-0 NTP AT 2B Acrolein (2-Propenal) 107-02-8 AT Acetamide 126850-14-4 2B 2-Acetylaminofluorene 53-96-3 NTP ORC Acrylamide 79-06-6 NTP 2B Acrylyl Chloride 814-68-6 AT Acrylonitrile 107-13-1 NTP ORC 2B Adriamycin 23214-92-8 NTP 2A Aflatoxins 1402-68-2 NTP 1 Allylamine 107-11-9 AT Alkylaluminums varies AT Allyl Chloride 107-05-1 AT ortho-Aminoazotoluene 97-56-3 NTP 2B para-aminoazobenzene 60-09-3 2B 4-Aminobiphenyl 92-67-1 NTP ORC 1 1-Amino-2-Methylanthraquinone 82-28-0 NTP (2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole) 67730-11-4 2B -

Managing Diseases and Insects in Home Orchards J
Managing diseases and insects in home orchards J. W. Pscheidt, H. Stoven, A. Thompson, B. Edmunds, N. Wiman, and R. Hilton In this guide, you can learn best pest management practices for your home Contents orchards. Suggested materials and times of application should have activity Table 1. Home garden/small orchard on the indicated pest. There are many fungicides and insecticides that are products ........................ 2 Importance of controlling diseases effective for managing the diseases and insects listed on the label when used and insects in commercial fruit according to the label directions. For more information, see the PNW Pest districts ......................... 3 Management Handbooks, at https://pnwhandbooks.org. Applying pesticides safely ......... 3 The best way to manage diseases and insects in your orchard is to combine Managing diseases and insects methods. Along with using pesticides, there are cultural and biological without using pesticides .......... 4 Apples .......................... 5 practices also that can help prevent or manage diseases and insects (see Pears ........................... 7 page 4). Pesticide timing and thorough spray coverage are the keys to good Peaches and Nectarines .......... 9 pest management. For good coverage, wet the leaves, twigs, and branches Apricots ........................10 thoroughly. (Note: This can be difficult with hand sprayers.) When you Cherries ........................11 use wettable powders, be sure to shake or stir the spray mix often during Prunes and Plums ...............13 application because the powders tend to settle at the bottom of the spray Walnuts ........................14 container after mixing. Hazelnuts (Filberts) .............14 To avoid excess chemical residues, be sure to use the correct rate and Moss and lichen .................15 proper interval between the last spray and harvest, as shown on the label. -

August, 1988 CHLOROTHALONIL Health Advisory Office of Drinking
August, 1988 CHLOROTHALONIL Health Advisory Office of Drinking Water U.S. Environmental Protection Agency I. INTRODUCTION The Health Advisory (HA) Program, sponsored by the Office of Drinking Water (ODW), provides information on the health effects, analytical methodology and treatment technology that would be useful in dealing with the contamination of drinking water. Health Advisories describe nonregulatory concentrations of drinking water contaminants at which adverse health effects would not be anticipated to occur over specific exposure durations. Health Advisories contain a margin of safety to protect sensitive members of the population. Health Advisories serve as informal technical guidance to assist Federal, State and local officials responsible for protecting public health when emergency spills or contamination situations occur. They are not to be construed as legally enforceable Federal standards. The HAs are subject to change as new information becomes available. Health Advisories are developed for one-day, ten-day, longer-term (approximately 7 years, or 10% of an individual’s lifetime) and lifetime exposures based on data describing noncarcinogenic end points of toxicity. For those substances that are known or probable human carcinogens, according to the Agency classification scheme (Group A or B), Lifetime HAs are not recommended. The chemical concentration values for Group A or B carcinogens are correlated with carcinogenic risk estimates by employing a cancer potency (unit risk) value together with assumptions for lifetime exposure and the consumption of drinking water. The cancer unit risk is usually derived from the linear multistage model with 95% upper confidence limits. This provides a low-dose estimate of cancer risk to humans that is considered unlikely to pose a carcinogenic risk in excess of the stated values. -

Chlorothalonil
Common Name: CHLOROTHALONIL CAS Number: 1897-45-6 DOT Number: UN 2588 RTK Substance number: 0415 DOT Hazard Class: 6.1 (Toxic) Date: April 1998 Revision: June 2005 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Chlorothalonil can affect you when breathed in. No occupational exposure limits have been established for * Chlorothalonil should be handled as a CARCINOGEN-- Chlorothalonil. This does not mean that this substance is not WITH EXTREME CAUTION. harmful. Safe work practices should always be followed. * Contact can irritate the skin and eyes. * Breathing Chlorothalonil can irritate the nose, throat and * Chlorothalonil may be a CARCINOGEN in humans. lungs causing cough, phlegm and/or tightness in the chest. There may be no safe level of exposure to a carcinogen, so * Repeated overexposure may cause nosebleeds and skin all contact should be reduced to the lowest possible level. rash. * Chlorothalonil may affect the kidneys. WAYS OF REDUCING EXPOSURE * Enclose operations and use local exhaust ventilation at the IDENTIFICATION site of chemical release. If local exhaust ventilation or Chlorothalonil is a white, odorless crystalline (sand-like) enclosure is not used, respirators should be worn. solid which may be found in a liquid formulation. It is used * Wear protective work clothing. as a fungicide for plants and crops. * Wash thoroughly immediately after exposure to Chlorothalonil and at the end of the workshift. REASON FOR CITATION * Post hazard and warning information in the work area. In * Chlorothalonil is on the Hazardous Substance List addition, as part of an ongoing education and training because it is cited by DOT, DEP, IARC, IRIS and EPA. effort, communicate all information on the health and * This chemical is on the Special Health Hazard Substance safety hazards of Chlorothalonil to potentially exposed List because it is a CARCINOGEN. -

Chlorothalonil: Reregistration Eligibility
United States Prevention, Pesticides EPA 738-R-99-004 Environmental Protection And Toxic Substances April 1999 Agency (7508C) Reregistration Eligibility Decision (RED) Chlorothalonil UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES CERTIFIED MAIL Dear Registrant: I am pleased to announce that the Environmental Protection Agency has completed its reregistration eligibility review and decisions on the pesticide chemical case 0097 for the active ingredient chlorothalonil. The enclosed Reregistration Eligibility Decision (RED), which was approved on September 30, 1998, contains the Agency's evaluation of the data base of this chemical, its conclusions of the potential human health and environmental risks of the current product uses, and its decisions and conditions under which these uses and products will be eligible for reregistration. The RED includes the data and labeling requirements for products for reregistration. It also includes requirements for additional data (generic) on the active ingredient to confirm the risk assessments. To assist you with a proper response, read the enclosed document entitled "Summary of Instructions for Responding to the RED.” This summary also refers to other enclosed documents which include further instructions. You must follow all instructions and submit complete and timely responses. The first set of required responses is due 90 days from the receipt of this letter. The second set of required responses is due 8 months from the date of this letter. Complete and timely responses will avoid the Agency taking the enforcement action of suspension against your products. Please note that the Food Quality Protection Act of 1996 (FQPA) became effective on August 3, 1996, amending portions of both pesticide law (FIFRA) and the food and drug law (FFDCA). -

MATERIAL SAFETY DATA SHEET Syngenta Crop
MATERIAL SAFETY DATA SHEET Syngenta Crop Protection, LLC In Case of Emergency, Call Post Office Box 18300 1-800-888-8372 Greensboro, NC 27419 1. PRODUCT IDENTIFICATION Product Name: QUADRIS OPTI Product No.: A13666B EPA Signal Word: Warning Active Ingredient(%): Azoxystrobin (4.6%) CAS No.: 131860-33-8 Chemical Name: Methyl (E)-2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3-methoxyacrylate Chemical Class: A Beta-Methyoxyacrylate Fungicide Active Ingredient(%): Chlorothalonil (46.0%) CAS No.: 1897-45-6 Chemical Name: Tetrachloroisophthalonitrile Chemical Class: Chlorinated Benzonitrile Fungicide EPA Registration Number(s): 100-1171 Section(s) Revised: 9, 14 2. HAZARDS IDENTIFICATION Health and Environmental Harmful if swallowed. Irritating to eyes. May cause an allergic skin reaction. Hazardous Decomposition Products None known. Physical Properties Appearance: Light yellow to greyish yellow liquid Odor: Not characteristic; weak Unusual Fire, Explosion and Reactivity Hazards During a fire, irritating and possibly toxic gases may be generated by thermal decomposition or combustion. 3. COMPOSITION/INFORMATION ON INGREDIENTS OSHA ACGIH NTP/IARC/OSHA Material PEL TLV Other Carcinogen Propylene Glycol Not Established Not Established 10 mg/m³ TWA **** No Azoxystrobin (4.6%) Not Established Not Established 2 mg/m³ TWA *** No Chlorothalonil (46.0%) Not Established Not Established 0.1 mg/m³ TWA *** IARC Group 2B *** Syngenta Occupational Exposure Limit (OEL) **** Recommended by AIHA (American Industrial Hygiene Association) Ingredients not precisely identified are proprietary or non-hazardous. Values are not product specifications. Syngenta Hazard Category: C, S 4. FIRST AID MEASURES Product Name: QUADRIS OPTI Page: 1 Have the product container, label or Material Safety Data Sheet with you when calling Syngenta (800-888-8372), a poison contol center or doctor, or going for treatment. -

Chlorothalonil
CHLOROTHALONIL This substance was considered by previous working groups, in 1982 (IARC, 1983) and 1987 (IARC, 1987). Since that time, new data have become available, and these have been incorporated into the monograph and taken into consideration in the present evaluation. 1. Exposure Data 1.1 Chemical and physical data 1.1.1 Nomenclature Chem. Abstr. Serv. Reg. No.: 1897-45-6 Deleted CAS Reg. Nos: 37223-69-1; 101963-73-9 Chem. Abstr. Name: 2,4,5,6-Tetrachloro-1,3-benzenedicarbonitrile IUPAC Systematic Name: Tetrachloroisophthalonitrile Synonyms: 1,3-Dicyanotetrachlorobenzene; tetrachlorobenzene-1,3-dicarbonitrile; 2,4,5,6-tetrachloro-1,3-dicyanobenzene; 2,4,5,6-tetrachloroisophthalonitrile; 2,4,5,6- tetrachloro-1,3-isophthalonitrile 1.1.2 Structural and molecular formulae and relative molecular mass CN Cl Cl Cl CN Cl C8Cl4N2 Relative molecular mass: 265.91 1.1.3 Chemical and physical properties of the pure substance (a) Description: White, crystalline solid (National Library of Medicine, 1998a) (b) Boiling-point: 350°C (Lide, 1997) (c) Melting-point: 250°C (Lide, 1997) (d) Density: 1.7 g/cm3 at 25°C (Lide, 1997) (e) Solubility: Insoluble in water; slightly soluble in acetone and cyclohexane (Lide, 1997) (f ) Volatility: Vapour pressure, < 1 Pa at 40°C (National Toxicology Program, 1991) –183– 184 IARC MONOGRAPHS VOLUME 73 (g) Octanol/water partition coefficient (P): log P, 2.90 (Hansch et al., 1995) (h) Conversion factor: mg/m3 = 10.88 × ppm 1.2 Production and use Chlorothalonil has been produced commercially since 1969 (WHO, 1996). Information available in 1995 indicated that chlorothalonil was produced in China and Italy (Chemical Information Services, 1995). -

Home Fruit Spray Guide
Fruits & Nuts includes : Home Fruit disease and insect control Home Fruit Spray Guide Tree Fruit diseases Small Fruit diseases Commercial Pecan diseases Homeowner Pecan Spray Guide Disease and Insect Control in Home Fruit Plantings Steve Bost, Professor; Karen Vail, Professor; Patricia Barnwell, Extension Program Assistant III and Frank Hale, Professor Entomology and Plant Pathology Growing fruit in the home garden can be an interesting, fun Rates of product application are not provided in these and rewarding hobby. Many novices dream of plucking perfect charts, because of the diversity of product concentrations fruit off trees in their yards. This does not happen without a offered. The product labels give the rates; follow them. The great deal of work. Control of pests (insects and diseases) is label rates are expressed as amount per gallon of water. The an integral part of the care necessary to achieve good results. following table can be used to determine the amount of spray This publication provides guidelines for spraying home fruit mixture needed. plantings, but good pest control is not possible if spraying is the only action taken. Cultural practices such as pruning, sanitation, Amount of spray needed for each application variety selection and selecting open, sunny sites for planting Gallon per tree are necessary for good pest control. Specific cultural practices Height in feet Spread in feet per application for each type of fruit are provided with the spray charts. 5 to 8 3 to 6 ½ to 1 How to Use the Spray Schedules 8 to 10 4 to 8 1 to 2 Most fungicide (disease control product) and some 10 to 15 8 to 15 3 to 5 insecticide (insect control product) applications are effective only if applied preventatively, because it is not possible to Protect Pollinating Insects control the pest satisfactorily after the fact.