Insulin–Insr Signaling Drives Multipotent Progenitor Differentiation Toward Lymphoid Lineages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DNA Damage and the Activation of the P53 Pathway Mediate Alterations in Metabolic and Secretory Functions of Adipocytes
3062 Diabetes Volume 65, October 2016 Bastien Vergoni,1,2 Pierre-Jean Cornejo,1,2 Jérôme Gilleron,1,2 Mansour Djedaini,1,2 Franck Ceppo,1,2 Arnaud Jacquel,2,3 Gwennaelle Bouget,1,2 Clémence Ginet,1,2 Teresa Gonzalez,1,2,4 Julie Maillet,5,6,7 Véronique Dhennin,5,6,7 Marie Verbanck,5,6,7 Patrick Auberger,2,3 Philippe Froguel,5,6,7,8 Jean-François Tanti,1,2 and Mireille Cormont1,2 DNA Damage and the Activation of the p53 Pathway Mediate Alterations in Metabolic and Secretory Functions of Adipocytes Diabetes 2016;65:3062–3074 | DOI: 10.2337/db16-0014 Activation of the p53 pathway in adipose tissue contributes Insulin resistance is associated with obesity and is a major to insulin resistance associated with obesity. However, the risk factor for type 2 diabetes. Adipose tissue (AT) plays mechanisms of p53 activation and the effect on adipocyte an important role in glucose and lipid homeostasis (1,2), functions are still elusive. Here we found a higher level of and AT dysfunction associated with obesity has emerged DNA oxidation and a reduction in telomere length in adipose as a critical event for the development of insulin resis- tissueofmicefedahigh-fatdietandanincreaseinDNA tance. Low-grade inflammation and oxidative and hypoxic damage and activation of the p53 pathway in adipocytes. stresses both develop in obese AT and are involved in the Interestingly, hallmarks of chronic DNA damage are visible alteration of adipocyte functions (3–6). Therefore, activa- at the onset of obesity. Furthermore, injection of lean mice tion of stress-signaling pathways in adipocytes is critically with doxorubicin, a DNA damage-inducing drug, increased involved in AT dysfunction and insulin resistance (7–10). -

Selective Insulin Signaling Through a and B Insulin Receptors Regulates Transcription of Insulin and Glucokinase Genes in Pancreatic  Cells
Molecular Cell, Vol. 7, 559±570, March, 2001, Copyright 2001 by Cell Press Selective Insulin Signaling through A and B Insulin Receptors Regulates Transcription of Insulin and Glucokinase Genes in Pancreatic  Cells Barbara Leibiger,*§ Ingo B. Leibiger,*§k ceptors as the primary target, include signaling via mito- Tilo Moede,* Sabine Kemper,* gen-activated protein (MAP) kinases and phosphoinosi- Rohit N. Kulkarni,² C. Ronald Kahn,² tol-3 kinase (PI3K). The insulin receptor (IR), the first Lina Moitoso de Vargas,³ and Per-Olof Berggren* step in these cascades, exists in two isoforms as a result *The Rolf Luft Center for Diabetes Research of alternative mRNA splicing of the 11th exon of the insulin Department of Molecular Medicine proreceptor transcript (Seino et al., 1989). The A type Karolinska Institutet (IR-A), or Ex11Ϫ (Ullrich et al., 1985), lacks whereas the S-171 76 Stockholm B type (IR-B), or Ex11ϩ (Ebina et al., 1985), contains Sweden the respective sequence coding for 12 amino acids in ² Research Division the C terminus of the ␣ chain of the receptor. To date, Joslin Diabetes Center and no insulin-induced effect has been reported that dis- Department of Medicine criminates signaling via A- and B-type receptors. In fact, Harvard Medical School the functional significance of these IR isoforms remains Boston, Massachusetts 02215 unclear. ³ Department of Medicine Recent studies have shown that the insulin-producing New England Medical Center and pancreatic  cell is a target for insulin action, with insulin Tufts University School of Medicine effects on transcription, translation, Ca2ϩ flux, and exo- Boston, Massachusetts 02111 cytosis (Leibiger et al., 1998a, 2000; Xu and Rothenberg, 1998; Xu et al., 1998; Aspinwall et al., 1999; Kulkarni et al., 1999a). -

Recombinant Human Thrombopoietin Promotes Hematopoietic
www.nature.com/scientificreports OPEN Recombinant human thrombopoietin promotes hematopoietic reconstruction after Received: 08 February 2015 Accepted: 06 July 2015 severe whole body irradiation Published: 25 September 2015 Chao Wang1,3,*, Bowen Zhang1,2,*, Sihan Wang1,2, Jing Zhang1,2, Yiming Liu1,2, Jingxue Wang1,2, Zeng Fan1,2, Yang Lv1,2, Xiuyuan Zhang1,2, Lijuan He1,2, Lin Chen1,2, Huanzhang Xia3, Yanhua Li1,2 & Xuetao Pei1,2 Recombinant human thrombopoietin (rHuTPO) is a drug that is used clinically to promote megakaryocyte and platelet generation. Here, we report the mitigative effect of rHuTPO (administered after exposure) against severe whole body irradiation in mice. Injection of rHuTPO for 14 consecutive days following exposure significantly improved the survival rate of lethally irradiated mice. RHuTPO treatment notably increased bone marrow cell density and LSK cell numbers in the mice after sub-lethal irradiation primarily by promoting residual HSC proliferation. In lethally irradiated mice with hematopoietic cell transplantation, rHuTPO treatment increased the survival rate and enhanced hematopoietic cell engraftment compared with the placebo treatment. Our observations indicate that recombinant human TPO might have a therapeutic role in promoting hematopoietic reconstitution and HSC engraftment. Accidental ionizing radiation exposure induces vital organ dysfunction syndromes in healthy individuals in radiological scenarios. The hematopoietic system is a radiosensitive organ that is highly suscepti- ble to damage1–3, and such damage can result in death. Clinically, radiation therapy or chemotherapy for patients with malignant diseases often leads to serious hematopoietic system damage. Modulation of the hematopoietic stem cell (HSC) population is considered to be key for realizing long-term sur- vival of patients. -

PSM, a Mediator of PDGF-BB-, IGF-I-, and Insulin-Stimulated Mitogenesis
Oncogene (2000) 19, 39 ± 50 ã 2000 Macmillan Publishers Ltd All rights reserved 0950 ± 9232/00 $15.00 www.nature.com/onc PSM, a mediator of PDGF-BB-, IGF-I-, and insulin-stimulated mitogenesis Heimo Riedel*,1,2, Nasim Yousaf1, Yuyuan Zhao4, Heping Dai1,3, Youping Deng1 and Jian Wang1 1Department of Biological Sciences, Wayne State University, Detroit, Michigan, MI 48202, USA; 2Member, Barbara Ann Karmanos Cancer Institute, Detroit, Michigan, MI 48201, USA PSM/SH2-B has been described as a cellular partner of Introduction the FceRI receptor, insulin receptor (IR), insulin-like growth factor-I (IGF-I) receptor (IGF-IR), and nerve The platelet-derived growth factor (PDGF) family growth factor receptor (TrkA). A function has been represents key mitogenic and chemotactic factors that proposed in neuronal dierentiation and development but have been exploited by the Simian sarcoma virus v-sis its role in other signaling pathways is still unclear. To oncogene which results in altered PDGF expression further elucidate the physiologic role of PSM we have (Water®eld et al., 1983). Normal cellular PDGF identi®ed additional mitogenic receptor tyrosine kinases appears in three distinct isoforms as any combination as putative PSM partners including platelet-derived of the PDGF A and B chains which act in paracrine growth factor (PDGF) receptor (PDGFR) beta, hepato- and autocrine mechanisms (Heldin, 1993). PDGF cyte growth factor receptor (Met), and ®broblast growth receptor (PDGFR) signal transduction appears to be factor receptor. We have mapped Y740 as a site of a largely membrane delineated event (Hughes et al., PDGFR beta that is involved in the association with 1996). -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

Structure of the Tie2 RTK Domain: Self-Inhibition by the Nucleotide Binding Loop, Activation Loop, and C-Terminal Tail
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Structure, Vol. 8, 1105±1113, November, 2000, 2000 Elsevier Science Ltd. All rights reserved. PII S0969-2126(00)00516-5 Structure of the Tie2 RTK Domain: Self-Inhibition by the Nucleotide Binding Loop, Activation Loop, and C-Terminal Tail Lisa M. Shewchuk,* Anne M. Hassell, Byron Ellis, binding domain and an intracellular kinase domain. Binding of W. D. Holmes, Roderick Davis, Earnest L. Horne, extracellular ligand is believed to promote dimerization, which Sue H. Kadwell, David D. McKee, leads to autophosphorylation and activation of the kinase do- and John T. Moore main [reviewed in 15]. Stringent regulation of the phosphoryla- GlaxoWellcome tion state and activity of the Tie2 kinase domain is crucial to Research Triangle Park, North Carolina 27709 normal vasculature development and maintenance. Tie2 activ- ity is precisely regulated by the opposing actions of agonistic and antagonistic extracellular ligands [16±18]. Tie2 activation Summary requires autophosporylation in response to binding its agonists angiopoietin 1 (Ang1) and Ang4, whereas inactivation occurs Background: Angiogenesis, the formation of new vessels from in response to Ang2 and Ang3. Tie2 mutations, which result the existing vasculature, is a critical process during early devel- in ligand-independent and enhanced autophosphorylation, opment as well as in a number of disease processes. Tie2 (also cause hereditary venous malformations [19, 20]. Conversely, known as Tek) is an endothelium-specific receptor tyrosine transgenic mice that express a kinase-inactive form of Tie2 or kinase involved in both angiogenesis and vasculature mainte- Tie2 null mice die in utero due to defects in their microvascula- nance. -
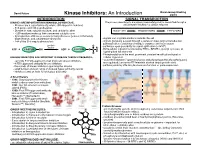
Kinase Inhibitors: an Introduction
David Peters Baran Group Meeting Kinase Inhibitors: An Introduction 2/2/19 INTRODUCTION SIGNAL TRANSDUCTION KINASES ARE IMPORTANT IN HUMAN BIOLOGY/DISEASE: The process describing how a signal (chemical/physical) is transmitted through a - Kinases are a superfamily of proteins (5th largest in humans) cell ultimately resulting in a cellular response - 518 genes and 106 pseudogenes - Diverse in size, subunit structure, and cellular location RECEPTOR TRANSDUCERS EFFECTORS - ~260 residues make up their conserved catalytic core - dysregulation of kinases occurs in many diseases (cancer, inflamatory, degenerative, and autoimmune diseases) - signals can originate inside or outside the cell - 244 of the 518 map to disease loci - signals generally passed through a series of steps (signal transduction pathway) often consisting of multiple enzymes and messengers protein O - pathways open possibility for signal aplification (>1x106) kinase ATP + PROTEIN OH ADP + PROTEIN O P O - Extracellular signals transduced by RTKs, GPCR’s, guanlyl cyclases, or ligand-gated Ion channels O - Phosphorylation is the most prominent covalent modification/signal in KINASES INHIBITORS ARE IMPORTANT IN DISEASE THERAPY/RESEARCH: cellular regulation - currently 51 FDA approved small molecule kinase inhibitors - “converter enzymes” (protein kinases and phosphoprotein phosphorlyases) - 4 FDA approved antibody kinase inhibitors are regulated; conserve ATP/maintain desired target protein state - thousands of known inhibitors spanning the kinome - pathway ends by affecting biomolecule -

LCZ696) Compared to Valsartan Attenuates Hepatotoxicity in STZ-Induced Hyperglycemic Rats Faleh Alqahtani#, Mohamed Mohany#, Abdullah F Alasmari, Ahmed Z
Int. J. Med. Sci. 2020, Vol. 17 3098 Ivyspring International Publisher International Journal of Medical Sciences 2020; 17(18): 3098-3106. doi: 10.7150/ijms.49373 Research Paper Angiotensin II receptor Neprilysin inhibitor (LCZ696) compared to Valsartan attenuates Hepatotoxicity in STZ-induced hyperglycemic rats Faleh Alqahtani#, Mohamed Mohany#, Abdullah F Alasmari, Ahmed Z. Alanazi, Osamah M. Belali, Mohammed M Ahmed, and Salim S Al-Rejaie Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, P.O. Box 55760, Riyadh – 1145, Saudi Arabia. #These authors contributed equally to this work. Corresponding author: E-mail: [email protected]; Tel.: +966114677178. © The author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). See http://ivyspring.com/terms for full terms and conditions. Received: 2020.06.11; Accepted: 2020.10.09; Published: 2020.10.22 Abstract Background and objectives: Although diabetic-induced hepatotoxicity is less common, it can be included in the list of target organ pathologies associated with diabetes. This study aimed to investigate the potential therapeutic role of sacubitril/valsartan (LCZ696) in modulating oxidative and inflammatory injuries and liver fibrosis in STZ-induced hyperglycemic rats in comparison to valsartan alone. Materials and Methods: Following the induction of diabetes using a single dose of streptozotocin (STZ), STZ-induced hyperglycemic animals were administered LCZ696 or valsartan for 6 weeks. Glucose, transaminases, lipid profile, tumor necrosis factor-alpha (TNF-α), interleukin 1 beta (IL-1β), and interleukin - 6 (IL-6), were estimated using the obtained serum. Oxidative stress biomarkers including thiobarbituric acid reactive substances (TBARS), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST) were measured in the liver homogenate. -

The Insulin and IGF-1 Receptors
The insulin and IGF-1 receptors Author: Pierre De Meyts Author title: MD, PHD, F.A.C.E. A bit of history The birth of the receptor concept dates back to the early work of John Newport Langley (1852-1925), a Cambridge physiologist, who postulated in 1905 that a "receptive substance" on the surface of skeletal muscle mediated the action of nicotine (1). At about the same time, Paul Ehrlich (1854-1915), a German immunologist who was the founding father of chemotherapy, came up with a "side chain theory" of cell receptors to explain the selectivity of immune reactions, winning him the Nobel Prize in Physiology or Medicine in 1908. His famous adage "Corpora non agunt nisi fixata" ("Substances do not act unless they are bound") is an elegant and concise early statement of the receptor theory (2). The receptor concept was put on more solid ground with the seminal 1948 paper of Raymond P. Ahlquist (1914-1983), an American pharmacologist of Swedish descent at the Medical College of Georgia, who proposed that the excitatory and inhibitory effects of adrenotropic agents were mediated by two separate receptors which he termed a and b (3). The receptors would however remain hypothetical entities until the late 60's, when direct methods to study their biochemistry were developed. The concept that insulin exerts its effects by acting at the membrane of target cells was proposed over 60 years ago by Rachmiel Levine (1910-1991), considered by many as one of the founding fathers of modern diabetology, then working at Walter Reese Hospital in Chicago. -

Five Mutant Alleles of the Insulin Receptor Gene in Patients with Genetic Forms of Insulin Resistance
Five mutant alleles of the insulin receptor gene in patients with genetic forms of insulin resistance. T Kadowaki, … , P Gorden, S I Taylor J Clin Invest. 1990;86(1):254-264. https://doi.org/10.1172/JCI114693. Research Article The nucleotide sequence was determined for all 22 exons of the insulin receptor gene from three patients with genetic syndromes associated with extreme insulin resistance. In all three patients, insulin resistance was caused by decreased insulin binding to the cell surface. The patient with leprechaunism (leprechaun/Winnipeg) came from a consanguineous pedigree and was homozygous for a missense mutation substituting arginine for His209 in the alpha-subunit of the insulin receptor. The other two patients were both compound heterozygotes with a nonsense mutation in one allele of the insulin receptor gene, and a missense mutation in the other allele. In the patient with the Rabson-Mendenhall syndrome (patient RM-1), the missense mutation substituted lysine for Asn15 in the alpha-subunit. In the patient with type A extreme insulin resistance (patient A-1), the missense mutation substituted serine for Asn462 in the alpha-subunit. Both nonsense mutations markedly reduced the levels of insulin receptor mRNA transcribed from the alleles with the nonsense mutation as compared to the transcripts from the other allele. The reduction in the level of mRNA would be predicted to greatly reduce the rate at which the truncated receptors would be synthesized. Furthermore, the truncated receptors would be severely impaired in their ability to mediate insulin action. Find the latest version: https://jci.me/114693/pdf Five Mutant Alleles of the Insulin Receptor Gene in Patients with Genetic Forms of Insulin Resistance Takashi Kadowaki,* Hiroko Kadowaki,* Matthew M. -

The Insulin Receptor: a Prototype for Dimeric, Allosteric Membrane Receptors?
Review The insulin receptor: a prototype for dimeric, allosteric membrane receptors? Pierre De Meyts Receptor Systems Biology Laboratory, Hagedorn Research Institute, Niels Steensens Vej 6, DK-2820, Gentofte, Denmark The recent crystallographic structure of the insulin receptor (IR) extracellular domain has brought us closer Glossary to ending several decades of speculation regarding the Allosteric: this refers to the regulation of the affinity or activity of a ligand stoichiometry and mechanism of insulin-receptor bind- binding to a protein (e.g. enzyme or receptor) by another ligand binding to a ing and negative cooperativity. It supports a bivalent different site than the ligand of reference (referred to as the orthosteric-binding site). The name comes from the Greek allos (meaning ‘other’) and stereos crosslinking model whereby two sites on the insulin (meaning ‘space’). It was initially used in the restricted sense of the Monod– molecule alternately crosslink two partial-binding sites Wyman–Changeux model of concerted ligand-induced conformational and on each insulin-receptor half. Ligand-induced or -stabil- affinity changes in proteins like haemoglobin, which can only generate positive cooperativity in ligand binding, but has recently been more loosely used in a ized receptor dimerization or oligomerization is a gen- broader sense equivalent to homotropic or heterotropic cooperativity, both eral feature of receptor tyrosine kinases (RTKs), in positive or negative, especially in the GPCR community. Cooperativity: a mechanism whereby binding of a ligand to one site of a addition to cytokine receptors, but the kinetic con- multimeric binding protein (like an enzyme or a receptor) affects the affinity of sequences of this mechanism have been less well stu- another ligand binding to another subunit of the same protein. -

Structural Basis and Genotype-Phenotype Correlations of INSR Mutations
Diabetes Page 2 of 62 Structural Basis and Genotype-phenotype Correlations of INSR Mutations Causing Severe Insulin Resistance Jun Hosoe1*, Hiroko Kadowaki2*, Fuyuki Miya1,3,4,5*, Katsuya Aizu6, Tomoyuki Kawamura7, Ichiro Miyata8, Kenichi Satomura9, Takeru Ito10, Kazuo Hara11, Masaki Tanaka12, Hiroyuki Ishiura12, Shoji Tsuji12, Ken Suzuki1, Minaka Takakura1, Keith A. Boroevich4, Tatsuhiko Tsunoda3,4,5, Toshimasa Yamauchi1, Nobuhiro Shojima1** & Takashi Kadowaki1** * J.H., H.K., and F.M. contributed equally to this work. ** Corresponding authors: Takashi Kadowaki, [email protected], and Nobuhiro Shojima, [email protected]. 1Department of Diabetes and Metabolic Diseases, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan 2Department of Pediatrics, Sanno Hospital, Tokyo, Japan. 3Department of Medical Science Mathematics, Medical Research Institute, Tokyo Medical and Dental University, Tokyo, Japan 4Laboratory for Medical Science Mathematics, RIKEN Center for Integrative Medical 1 Diabetes Publish Ahead of Print, published online August 1, 2017 Page 3 of 62 Diabetes Sciences, Yokohama, Japan 5CREST, JST, Tokyo, Japan 6Division of Endocrinology and Metabolism, Saitama Children's Medical Center, Saitama, Japan 7Department of Pediatrics, Osaka City University Graduate School of Medicine, Osaka, Japan 8Department of Pediatrics, The Jikei University School of Medicine, Tokyo, Japan 9Department of Pediatric Nephrology and Metabolism, Osaka Medical Center and Research Institute for Maternal and Child Health, Izumi, Japan 10Department of Pediatrics, Atsugi City Hospital, Kanagawa, Japan 11Department of Endocrinology and Metabolism, Saitama Medical Center, Jichi Medical University, Saitama, Japan 12Department of Neurology, Graduate School of Medicine, The University of Tokyo, Tokyo, Japan 2 Diabetes Page 4 of 62 Abstract The insulin receptor (INSR) gene was analyzed in four patients with severe insulin resistance, revealing 5 novel mutations and a deletion that removed exon 2.