Pim1 Kinase Regulates C-Kit Gene Translation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insulin–Insr Signaling Drives Multipotent Progenitor Differentiation Toward Lymphoid Lineages
Article Insulin–InsR signaling drives multipotent progenitor differentiation toward lymphoid lineages Pengyan Xia,1* Shuo Wang,1* Ying Du,1 Guanling Huang,1,2 Takashi Satoh,3 Shizuo Akira,3 and Zusen Fan1 1Key Laboratory of Infection and Immunity of CAS, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China 2University of Chinese Academy of Sciences, Beijing 100049, China 3Department of Host Defense, Research Institute for Microbial Diseases (RIMD), Osaka University, Suita, Osaka 565-0871, Japan The lineage commitment of HSCs generates balanced myeloid and lymphoid populations in hematopoiesis. However, the un- derlying mechanisms that control this process remain largely unknown. Here, we show that insulin–insulin receptor (InsR) signaling is required for lineage commitment of multipotent progenitors (MPPs). Deletion of Insr in murine bone marrow causes skewed differentiation of MPPs to myeloid cells. mTOR acts as a downstream effector that modulates MPP differentiation. mTOR activates Stat3 by phosphorylation at serine 727 under insulin stimulation, which binds to the promoter of Ikaros, lead- ing to its transcription priming. Our findings reveal that the insulin–InsR signaling drives MPP differentiation into lymphoid lineages in early lymphopoiesis, which is essential for maintaining a balanced immune system for an individual organism. Hematopoiesis is the process of producing all compo- Insulin, as the primary anabolic hormone, modulates a nents of the blood system from hematopoietic stem cells variety of physiological processes, including growth, differ- (HSCs; Naik et al., 2013; Mendelson and Frenette, 2014; entiation, apoptosis, and synthesis and breakdown of lipid, Walter et al., 2015). HSCs are quiescent, self-renewable pro- protein, and glucose (Samuel and Shulman, 2012). -

Recombinant Human Thrombopoietin Promotes Hematopoietic
www.nature.com/scientificreports OPEN Recombinant human thrombopoietin promotes hematopoietic reconstruction after Received: 08 February 2015 Accepted: 06 July 2015 severe whole body irradiation Published: 25 September 2015 Chao Wang1,3,*, Bowen Zhang1,2,*, Sihan Wang1,2, Jing Zhang1,2, Yiming Liu1,2, Jingxue Wang1,2, Zeng Fan1,2, Yang Lv1,2, Xiuyuan Zhang1,2, Lijuan He1,2, Lin Chen1,2, Huanzhang Xia3, Yanhua Li1,2 & Xuetao Pei1,2 Recombinant human thrombopoietin (rHuTPO) is a drug that is used clinically to promote megakaryocyte and platelet generation. Here, we report the mitigative effect of rHuTPO (administered after exposure) against severe whole body irradiation in mice. Injection of rHuTPO for 14 consecutive days following exposure significantly improved the survival rate of lethally irradiated mice. RHuTPO treatment notably increased bone marrow cell density and LSK cell numbers in the mice after sub-lethal irradiation primarily by promoting residual HSC proliferation. In lethally irradiated mice with hematopoietic cell transplantation, rHuTPO treatment increased the survival rate and enhanced hematopoietic cell engraftment compared with the placebo treatment. Our observations indicate that recombinant human TPO might have a therapeutic role in promoting hematopoietic reconstitution and HSC engraftment. Accidental ionizing radiation exposure induces vital organ dysfunction syndromes in healthy individuals in radiological scenarios. The hematopoietic system is a radiosensitive organ that is highly suscepti- ble to damage1–3, and such damage can result in death. Clinically, radiation therapy or chemotherapy for patients with malignant diseases often leads to serious hematopoietic system damage. Modulation of the hematopoietic stem cell (HSC) population is considered to be key for realizing long-term sur- vival of patients. -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -
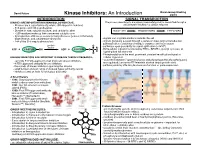
Kinase Inhibitors: an Introduction
David Peters Baran Group Meeting Kinase Inhibitors: An Introduction 2/2/19 INTRODUCTION SIGNAL TRANSDUCTION KINASES ARE IMPORTANT IN HUMAN BIOLOGY/DISEASE: The process describing how a signal (chemical/physical) is transmitted through a - Kinases are a superfamily of proteins (5th largest in humans) cell ultimately resulting in a cellular response - 518 genes and 106 pseudogenes - Diverse in size, subunit structure, and cellular location RECEPTOR TRANSDUCERS EFFECTORS - ~260 residues make up their conserved catalytic core - dysregulation of kinases occurs in many diseases (cancer, inflamatory, degenerative, and autoimmune diseases) - signals can originate inside or outside the cell - 244 of the 518 map to disease loci - signals generally passed through a series of steps (signal transduction pathway) often consisting of multiple enzymes and messengers protein O - pathways open possibility for signal aplification (>1x106) kinase ATP + PROTEIN OH ADP + PROTEIN O P O - Extracellular signals transduced by RTKs, GPCR’s, guanlyl cyclases, or ligand-gated Ion channels O - Phosphorylation is the most prominent covalent modification/signal in KINASES INHIBITORS ARE IMPORTANT IN DISEASE THERAPY/RESEARCH: cellular regulation - currently 51 FDA approved small molecule kinase inhibitors - “converter enzymes” (protein kinases and phosphoprotein phosphorlyases) - 4 FDA approved antibody kinase inhibitors are regulated; conserve ATP/maintain desired target protein state - thousands of known inhibitors spanning the kinome - pathway ends by affecting biomolecule -

S42003-021-02215-W.Pdf
ARTICLE https://doi.org/10.1038/s42003-021-02215-w OPEN Actin cytoskeleton deregulation confers midostaurin resistance in FLT3-mutant acute myeloid leukemia Andoni Garitano-Trojaola1, Ana Sancho 2,3, Ralph Götz4, Patrick Eiring4, Susanne Walz5, Hardikkumar Jetani1, Jesus Gil-Pulido6, Matteo Claudio Da Via 1, Eva Teufel1, Nadine Rhodes1, Larissa Haertle 1, Estibaliz Arellano-Viera1, Raoul Tibes1, Andreas Rosenwald7, Leo Rasche1, Michael Hudecek 1, ✉ Markus Sauer 4, Jürgen Groll 2, Hermann Einsele 1, Sabrina Kraus1,8 & Martin K. Kortüm 1,8 The presence of FMS-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) is one of 1234567890():,; the most frequent mutations in acute myeloid leukemia (AML) and is associated with an unfavorable prognosis. FLT3 inhibitors, such as midostaurin, are used clinically but fail to entirely eradicate FLT3-ITD + AML. This study introduces a new perspective and highlights the impact of RAC1-dependent actin cytoskeleton remodeling on resistance to midostaurin in AML. RAC1 hyperactivation leads resistance via hyperphosphorylation of the positive reg- ulator of actin polymerization N-WASP and antiapoptotic BCL-2. RAC1/N-WASP, through ARP2/3 complex activation, increases the number of actin filaments, cell stiffness and adhesion forces to mesenchymal stromal cells (MSCs) being identified as a biomarker of resistance. Midostaurin resistance can be overcome by a combination of midostaruin, the BCL-2 inhibitor venetoclax and the RAC1 inhibitor Eht1864 in midostaurin-resistant AML cell lines and primary samples, providing the first evidence of a potential new treatment approach to eradicate FLT3-ITD + AML. 1 Department of Internal Medicine II, University Hospital Würzburg, Würzburg, Germany. 2 Department of Functional Materials in Medicine and Dentistry and Bavarian Polymer Institute, University of Würzburg, Würzburg, Germany. -

Mouse CD Marker Chart Bdbiosciences.Com/Cdmarkers
BD Mouse CD Marker Chart bdbiosciences.com/cdmarkers 23-12400-01 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD1d CD1.1, CD1.2, Ly-38 Lipid, Glycolipid Ag + + + + + + + + CD104 Integrin b4 Laminin, Plectin + DNAX accessory molecule 1 (DNAM-1), Platelet and T cell CD226 activation antigen 1 (PTA-1), T lineage-specific activation antigen 1 CD112, CD155, LFA-1 + + + + + – + – – CD2 LFA-2, Ly-37, Ly37 CD48, CD58, CD59, CD15 + + + + + CD105 Endoglin TGF-b + + antigen (TLiSA1) Mucin 1 (MUC1, MUC-1), DF3 antigen, H23 antigen, PUM, PEM, CD227 CD54, CD169, Selectins; Grb2, β-Catenin, GSK-3β CD3g CD3g, CD3 g chain, T3g TCR complex + CD106 VCAM-1 VLA-4 + + EMA, Tumor-associated mucin, Episialin + + + + + + Melanotransferrin (MT, MTF1), p97 Melanoma antigen CD3d CD3d, CD3 d chain, T3d TCR complex + CD107a LAMP-1 Collagen, Laminin, Fibronectin + + + CD228 Iron, Plasminogen, pro-UPA (p97, MAP97), Mfi2, gp95 + + CD3e CD3e, CD3 e chain, CD3, T3e TCR complex + + CD107b LAMP-2, LGP-96, LAMP-B + + Lymphocyte antigen 9 (Ly9), -

CD135) Expression in Pediatric Acute Myeloid Leukemia: a Report from the Children’S Oncology Group
Author Manuscript Published OnlineFirst on January 20, 2017; DOI: 10.1158/1078-0432.CCR-16-2353 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Disease Characteristics and Prognostic Implications of Cell Surface FLT3 Receptor (CD135) Expression in Pediatric Acute Myeloid Leukemia: A Report from the Children’s Oncology Group Katherine Tarlock1,2, Todd A. Alonzo3,4, Michael R. Loken5, Robert B. Gerbing3, Rhonda E. Ries,2 Richard Aplenc6, Lillian Sung7, Susana C. Raimondi8, Betsy A. Hirsch9, Samir B. Kahwash10, Amy McKenney11, E Anders Kolb12, Alan S. Gamis13 and Soheil Meshinchi1,2 1 Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle Washington; 2 Department of Pediatrics, Seattle Children’s Hospital, University of Washington, Seattle, Washington; 3 Children’s Oncology Group, Monrovia, California; 4 Keck School of Medicine, University of Southern California, Los Angeles, California; 5 Hematologics, Inc, Seattle, Washington 6 Division of Hematology/Oncology, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania; 7 Division of Haematology/Oncology, The Hospital for Sick Children, Toronto, Ontario; 8 St. Jude Children’s Research Hospital, Memphis, Tennessee; 9 Division of Laboratory Medicine, University of Minnesota Medical School, Minneapolis, Minnesota; 10 Nationwide Children’s Hospital, Columbus, Ohio; 11 Cleveland Clinic Foundation, Cleveland, Ohio; 12 Nemours/Alfred I duPont Hospital for Children, Wilmington, Delaware; 13 Children’s Mercy Hospitals and Clinics, Kansas City, Missouri Running title: CD135 Expression in pediatric AML Keywords: CD135, AML, FLT3 expression Financial Support: This work was supported by grants to the Children’s Oncology Group including U10CA098543 (Chair’s grant) and U10CA098413 (Statistical Center). -

Pathway Entry Into the T Lymphocyte Developmental Molecular Dissection of Prethymic Progenitor
The Journal of Immunology Molecular Dissection of Prethymic Progenitor Entry into the T Lymphocyte Developmental Pathway1 C. Chace Tydell,2 Elizabeth-Sharon David-Fung,2,3 Jonathan E. Moore, Lee Rowen,4 Tom Taghon,5 and Ellen V. Rothenberg6 Notch signaling activates T lineage differentiation from hemopoietic progenitors, but relatively few regulators that initiate this program have been identified, e.g., GATA3 and T cell factor-1 (TCF-1) (gene name Tcf7). To identify additional regulators of T cell specification, a cDNA library from mouse Pro-T cells was screened for genes that are specifically up-regulated in intrathymic T cell precursors as compared with myeloid progenitors. Over 90 genes of interest were iden- tified, and 35 of 44 tested were confirmed to be more highly expressed in T lineage precursors relative to precursors of B and/or myeloid lineage. To a remarkable extent, however, expression of these T lineage-enriched genes, including zinc finger transcription factor, helicase, and signaling adaptor genes, was also shared by stem cells (Lin؊Sca-1؉Kit؉CD27؊) and multipotent progenitors (Lin؊Sca-1؉Kit؉CD27؉), although down-regulated in other lineages. Thus, a major fraction of these early T lineage genes are a regulatory legacy from stem cells. The few genes sharply up-regulated between multipotent progenitors and Pro-T cell stages included those encoding transcription factors Bcl11b, TCF-1 (Tcf7), and HEBalt, Notch target Deltex1, Deltex3L, Fkbp5, Eva1, and Tmem131. Like GATA3 and Deltex1, Bcl11b, Fkbp5, and Eva1 were dependent on Notch/Delta signaling for induction in fetal liver precursors, but only Bcl11b and HEBalt were up-regulated between the first two stages of intrathymic T cell development (double negative 1 and double negative 2) corresponding to T lineage specification. -

Moguntinones—New Selective Inhibitors for the Treatment of Human Colorectal Cancer
Published OnlineFirst April 17, 2014; DOI: 10.1158/1535-7163.MCT-13-0224 Molecular Cancer Small Molecule Therapeutics Therapeutics Moguntinones—New Selective Inhibitors for the Treatment of Human Colorectal Cancer Annett Maderer1, Stanislav Plutizki2, Jan-Peter Kramb2, Katrin Gopfert€ 1, Monika Linnig1, Katrin Khillimberger1, Christopher Ganser2, Eva Lauermann2, Gerd Dannhardt2, Peter R. Galle1, and Markus Moehler1 Abstract 3-Indolyl and 3-azaindolyl-4-aryl maleimide derivatives, called moguntinones (MOG), have been selected for their ability to inhibit protein kinases associated with angiogenesis and induce apoptosis. Here, we characterize their mode of action and their potential clinical value in human colorectal cancer in vitro and in vivo. MOG-19 and MOG-13 were characterized in vitro using kinase, viability, and apoptosis assays in different human colon cancer (HT-29, HCT-116, Caco-2, and SW480) and normal colon cell lines (CCD-18Co, FHC, and HCoEpiC) alone or in combination with topoisomerase I inhibitors. Intracellular signaling pathways were analyzed by Western blotting. To determine their potential to inhibit tumor growth in vivo, the human HT-29 tumor xenograft model was used. Moguntinones prominently inhibit several protein kinases associated with tumor growth and metastasis. Specific signaling pathways such as GSK3b and mTOR downstream targets b were inhibited with IC50 values in the nanomolar range. GSK3 signaling inhibition was independent of KRAS, BRAF, and PI3KCA mutation status. While moguntinones alone induced apoptosis only in concentra- tions >10 mmol/L, MOG-19 in combination with topoisomerase I inhibitors induced apoptosis synergistically at lower concentrations. Consistent with in vitro data, MOG-19 significantly reduced tumor volume and weight in combination with a topoisomerase I inhibitor in vivo. -

Aberrant Bone Homeostasis in AML Is Associated with Activated Oncogenic FLT3-Dependent Cytokine Networks
cells Article Aberrant Bone Homeostasis in AML Is Associated with Activated Oncogenic FLT3-Dependent Cytokine Networks 1, 2, 3 3,4 5, Isabel Bär y, Volker Ast y, Daria Meyer , Rainer König , Martina Rauner z, 5, , 1, , Lorenz C. Hofbauer * z and Jörg P. Müller * z 1 Institute of Molecular Cell Biology, Center for Molecular Biomedicine (CMB), Jena University Hospital, 07745 Jena, Germany; [email protected] 2 Institute for Clinical Chemistry, Medical Faculty Mannheim, Heidelberg University, 69117 Heidelberg, Germany; [email protected] 3 Center for Infectious Diseases and Infection Control, Jena University Hospital, 07745 Jena, Germany; [email protected] (D.M.); [email protected] (R.K.) 4 Integrated Research and Treatment Center, Center for Sepsis Control and Care (CSCC), 07745 Jena, Germany 5 Department of Medicine III & Center for Healthy Aging, Technical University Dresden, 01069 Dresden, Germany; [email protected] * Correspondence: [email protected] (L.C.H.); [email protected] (J.P.M.); Tel.: +49-351-458-3173 (L.C.H.); +49-364-1939-5634 (J.P.M.) These authors joined first. y These authors equally contributed. z Received: 5 October 2020; Accepted: 5 November 2020; Published: 9 November 2020 Abstract: Acute myeloid leukaemia (AML) is a haematopoietic malignancy caused by a combination of genetic and epigenetic lesions. Activation of the oncoprotein FLT3 ITD (Fms-like tyrosine kinase with internal tandem duplications) represents a key driver mutation in 25–30% of AML patients. FLT3 is a class III receptor tyrosine kinase, which plays a role in cell survival, proliferation, and differentiation of haematopoietic progenitors of lymphoid and myeloid lineages. -

Spotlight on Midostaurin in the Treatment of FLT3-Mutated Acute Myeloid Leukemia and Systemic Mastocytosis: Design, Development, and Potential Place in Therapy
Spotlight on midostaurin in the treatment of FLT3-mutated acute myeloid leukemia and systemic mastocytosis: design, development, and potential place in therapy The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Weisberg, Ellen, Martin Sattler, Paul W Manley, and James D Griffin. 2018. “Spotlight on midostaurin in the treatment of FLT3- mutated acute myeloid leukemia and systemic mastocytosis: design, development, and potential place in therapy.” OncoTargets and therapy 11 (1): 175-182. doi:10.2147/OTT.S127679. http:// dx.doi.org/10.2147/OTT.S127679. Published Version doi:10.2147/OTT.S127679 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:34868830 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Journal name: OncoTargets and Therapy Article Designation: Review Year: 2018 Volume: 11 OncoTargets and Therapy Dovepress Running head verso: Weisberg et al Running head recto: Development of midostaurin for mutant FLT3-positive AML open access to scientific and medical research DOI: http://dx.doi.org/10.2147/OTT.S127679 Open Access Full Text Article REVIEW Spotlight on midostaurin in the treatment of FLT3-mutated acute myeloid leukemia and systemic mastocytosis: design, development, and potential place in therapy Ellen Weisberg1,2 Abstract: The Fms-like tyrosine kinase-3 (FLT3; fetal liver kinase-2; human stem cell tyrosine Martin Sattler1,2 kinase-1; CD135) is a class III receptor tyrosine kinase that is normally involved in regulating Paul W Manley3 the proliferation, differentiation, and survival of both hematopoietic cells and dendritic cells. -

A Wonder Drug for Targeted Cancer Therapy in Non-Small Cell Lung Cancer ⇑ Silky Bedi A, Shah A
Saudi Pharmaceutical Journal 26 (2018) 755–763 Contents lists available at ScienceDirect Saudi Pharmaceutical Journal journal homepage: www.sciencedirect.com Review A comprehensive review on Brigatinib – A wonder drug for targeted cancer therapy in non-small cell lung cancer ⇑ Silky Bedi a, Shah A. Khan b, Majed M. AbuKhader b, Perwez Alam c, Nasir A. Siddiqui c, Asif Husain a, a Department of Pharmaceutical Chemistry, School of Pharmaceutical Education and Research, Jamia Hamdard, New Delhi 110062, India b Department of Pharmacy, Oman Medical College, Muscat, Oman c Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia article info abstract Article history: The mortality rate in patients suffering from non-small cell lung cancer (NSCLC) is quite high. This type of Received 28 February 2018 cancer mainly occurs due to rearrangements in the anaplastic lymphoma kinase (ALK) gene which leads Accepted 18 April 2018 to form an oncogene of fused gene NPM-ALK. Brigatinib is recently approved by FDA in April 2017 as a Available online 20 April 2018 potent tyrosine kinase inhibitor (TKI) for the NSCLC therapy. In the present scenario, it is no less than a wonder drug because it is indicated for the treatment of advanced stages of metastatic ALK positive Keywords: NSCLC, a fatal disease to overcome the resistance of various other ALK inhibitors such as crizotinib, cer- ALK inhibitors itinib and alectinib. In addition to ALK, it is also active against multiple types of kinases such as ROS1, Brigatinib Insulin like growth factor-1Receptor and EGFR. It can be synthesized by using N-[2-methoxy-4-[4- Lung cancer Kinase (dimethylamino) piperidin-1-yl] aniline] guanidine and 2,4,5-trichloropyrimidine respectively in two dif- Lymphoma ferent ways.