GATA2 Mutant Disease Phenotypes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functions of the Mineralocorticoid Receptor in the Hippocampus By
Functions of the Mineralocorticoid Receptor in the Hippocampus by Aaron M. Rozeboom A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Cellular and Molecular Biology) in The University of Michigan 2008 Doctoral Committee: Professor Audrey F. Seasholtz, Chair Professor Elizabeth A. Young Professor Ronald Jay Koenig Associate Professor Gary D. Hammer Assistant Professor Jorge A. Iniguez-Lluhi Acknowledgements There are more people than I can possibly name here that I need to thank who have helped me throughout the process of writing this thesis. The first and foremost person on this list is my mentor, Audrey Seasholtz. Between working in her laboratory as a research assistant and continuing my training as a graduate student, I spent 9 years in Audrey’s laboratory and it would be no exaggeration to say that almost everything I have learned regarding scientific research has come from her. Audrey’s boundless enthusiasm, great patience, and eager desire to teach students has made my time in her laboratory a richly rewarding experience. I cannot speak of Audrey’s laboratory without also including all the past and present members, many of whom were/are not just lab-mates but also good friends. I also need to thank all the members of my committee, an amazing group of people whose scientific prowess combined with their open-mindedness allowed me to explore a wide variety of interests while maintaining intense scientific rigor. Outside of Audrey’s laboratory, there have been many people in Ann Arbor without whom I would most assuredly have gone crazy. -

Supplemental Material 1 (PDF)
Supplementary Figure 1. Murine and human GATA2 promoter alignment shows highly conserved regions. Brackets delimit the highly conserved regions in both genomes, as graphics obtained from ECR Browser show: large areas marked in red indicate highly similar intergenic sequences, repetitive sequences in green, exonic sequences in yellow, and intronic sequences in salmon color. Horizontal axis represents base positions in the genome and the vertical axis represents the percentage identity between the human and mouse genomes. GATA2 binding sites are indicated in red within the brackets, and they were located using the TFSearch, Match and MatInspector transcription factor binding site finder algorithms. Comparison of GATA2 binding sites between both genomes and several surrounding nucleotides is shown in the middle, where GATA2 binding site core sequences are shown in italic and underlined. Two GATA2 binding sites at -2.8 belonged to a highly conserved region spanning 389 bp, with 87.7% homology with the human GATA2 promoter. Similarly, other two GATA2 binding sites at -1.8 were located within a 282 bp region with 75.5% homology. Additionally, a third 142 bp region was taken into account containing the -2992 GATA2 binding site, which showed 68.3% homology. Supplementary Table I: List of reported germline GATA2 mutations and associated syndromes. Mutation (DNA)* Affected exon Mutation (protein) Mutation type Affected ZF Reference Cases/families Associated syndrome c.1-200_871+527del2033 4, 5 p.Met1_Ser290del In-frame deletion None 4,7,20,S1 2/1 -

The Title of the Dissertation
UNIVERSITY OF CALIFORNIA SAN DIEGO Novel network-based integrated analyses of multi-omics data reveal new insights into CD8+ T cell differentiation and mouse embryogenesis A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Bioinformatics and Systems Biology by Kai Zhang Committee in charge: Professor Wei Wang, Chair Professor Pavel Arkadjevich Pevzner, Co-Chair Professor Vineet Bafna Professor Cornelis Murre Professor Bing Ren 2018 Copyright Kai Zhang, 2018 All rights reserved. The dissertation of Kai Zhang is approved, and it is accept- able in quality and form for publication on microfilm and electronically: Co-Chair Chair University of California San Diego 2018 iii EPIGRAPH The only true wisdom is in knowing you know nothing. —Socrates iv TABLE OF CONTENTS Signature Page ....................................... iii Epigraph ........................................... iv Table of Contents ...................................... v List of Figures ........................................ viii List of Tables ........................................ ix Acknowledgements ..................................... x Vita ............................................. xi Abstract of the Dissertation ................................. xii Chapter 1 General introduction ............................ 1 1.1 The applications of graph theory in bioinformatics ......... 1 1.2 Leveraging graphs to conduct integrated analyses .......... 4 1.3 References .............................. 6 Chapter 2 Systematic -

5045.Full.Pdf
IFN Consensus Sequence Binding Protein (Icsbp) Is Critical for Eosinophil Development This information is current as Maja Milanovic, Grzegorz Terszowski, Daniela Struck, of September 28, 2021. Oliver Liesenfeld and Dirk Carstanjen J Immunol 2008; 181:5045-5053; ; doi: 10.4049/jimmunol.181.7.5045 http://www.jimmunol.org/content/181/7/5045 Downloaded from References This article cites 47 articles, 33 of which you can access for free at: http://www.jimmunol.org/content/181/7/5045.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2008 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology IFN Consensus Sequence Binding Protein (Icsbp) Is Critical for Eosinophil Development1 Maja Milanovic,2* Grzegorz Terszowski,2† Daniela Struck,2‡ Oliver Liesenfeld,‡ and Dirk Carstanjen3* IFN consensus sequence binding protein (Icsbp) (IFN response factor-8) is a hematopoietic transcription factor with dual functions in myelopoiesis and immunity. -
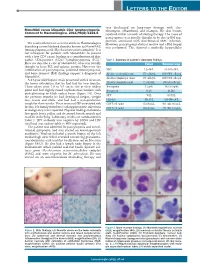
Letters to the Editor
LETTERS TO THE EDITOR was discharged on long-term therapy with clar - + MonoMAC versus idiopathic CD4 lymphocytopenia . ithromycin, ethambutol, and rifampin. His skin lesions Comment to Haematologica. 2011;96(8):1221-5 resolved within a month of starting therapy. The cause of pancytopenia was initially thought to be due to BM sup - pression associated with disseminated MAC infection. We read with interest a recent article in Haematologica However, pancytopenia did not resolve and a BM biopsy describing a novel defined disorder known as MonoMAC was performed. This showed a markedly hypocellular (monocytopenia with Mycobacterium avium complex). 1 It is not infrequent for patients with MonoMAC to present with a low CD4 count, leading to a consideration of idio - pathic CD4-positive (CD4 +) lymphocytopenia (ICL). 2 Table 1. Summary of patient’s laboratory findings. Here we describe a case of MonoMAC who was initially Patient Reference range thought to have ICL and aplastic anemia. However, the combination of pancytopenia, profound monocytopenia, WBC 1.2 ¥10 9/L 4.1-10.9 ¥10 9/L and bone marrow (BM) findings support a diagnosis of Absolute neutrophil count 876 cells/ mL 1400-6500 cells/ mL MonoMAC. Absolute lymphocyte count 144 cells/ mL 1200-3400 cells/ mL A 24-year old Hispanic male presented with 2 ulcers on the lower extremities that he had had for two months. Absolute monocyte count 12 cells/ mL 300-820 cells/ mL These ulcers were 1.0 to 3.5 cm in size at their widest Hemoglobin 5.2 g/dL 14.0-18.0 g/dL point and had slightly raised erythematous borders with Hematocrit 16.2% 40-54% pink-glistening to black eschar bases (Figure 1A). -

GATA2 Regulates the Erythropoietin Receptor in T(12;21) ALL
GATA2 regulates the erythropoietin receptor in t(12;21) ALL Gaine, M. E., Sharpe, D. J., Smith, J. S., Colyer, H., Hodges, V. M., Lappin, T. R., & Mills, K. I. (2017). GATA2 regulates the erythropoietin receptor in t(12;21) ALL. Oncotarget, 8(39), 66061-66074. https://doi.org/10.18632/oncotarget.19792 Published in: Oncotarget Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights © 2017 The Authors. This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:04. Oct. 2021 www.impactjournals.com/oncotarget/ Oncotarget, 2017, Vol. 8, (No. 39), pp: 66061-66074 Research Paper GATA2 regulates the erythropoietin receptor in t(12;21) ALL Marie E. -

And EZH2-Regulated Gene, Has Differential Roles in AR-Dependent and -Independent Prostate Cancer
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 6, No.4 RUNX1, an androgen- and EZH2-regulated gene, has differential roles in AR-dependent and -independent prostate cancer Ken-ichi Takayama1,2, Takashi Suzuki3, Shuichi Tsutsumi4, Tetsuya Fujimura5, Tomohiko Urano1,2, Satoru Takahashi6, Yukio Homma5, Hiroyuki Aburatani4 and Satoshi Inoue1,2,7 1 Department of Anti-Aging Medicine, The University of Tokyo, Bunkyo-ku, Tokyo, Japan 2 Department of Geriatric Medicine, The University of Tokyo, Bunkyo-ku, Tokyo, Japan 3 Department of Pathology, Tohoku University Graduate School of Medicine, Sendai, Miyagi, Japan 4 Genome Science Division, Research Center for Advanced Science and Technology (RCAST), The University of Tokyo, Meguro-ku, Tokyo, Japan 5 Department of Urology, Graduate School of Medicine, The University of Tokyo, Bunkyo-ku, Tokyo, Japan 6 Department of Urology, Nihon University School of Medicine, Itabashi-ku, Tokyo, Japan 7 Division of Gene Regulation and Signal Transduction, Research Center for Genomic Medicine, Saitama Medical University, Hidaka, Saitama, Japan Correspondence to: Satoshi Inoue, email: [email protected] Keywords: RUNX1, androgen receptor, EZH2, prostate cancer Received: October 02, 2014 Accepted: December 09, 2014 Published: December 10, 2014 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Androgen receptor (AR) signaling is essential for the development of prostate cancer. Here, we report that runt-related transcription factor (RUNX1) could be a key molecule for the androgen-dependence of prostate cancer. We found RUNX1 is a target of AR and regulated positively by androgen. -

Editorials & Perspectives
EDITORIALS & PERSPECTIVES Dendritic cell, monocyte, B and NK lymphoid deficiency defines the lost lineages of a new GATA-2 dependent myelodysplastic syndrome Venetia Bigley and Matthew Collin Institute of Cellular Medicine, Newcastle University, UK. E-mail: [email protected] doi:10.3324/haematol.2011.048355 (Related Original Article on page 1221) novel immunodeficiency syndrome has recently been International Prognostic Scoring System (IPSS). defined in young adults. Known variously as dendritic It is worth emphasizing at this point that DCML deficiency cell, monocyte, B and NK lymphoid (DCML deficien - patients may have significant risk to their health before addi - A1 2 2 cy), ‘autosomal dominant and sporadic monocytopenia’ or tional cytopenias evolve. Disseminated mycobacterial infec - ‘MonoMAC’ (monocytopenia with Mycobacterium avium com - tion, PAP and carcinoma in situ may all occur prior to the onset plex), 3 it is characterized by a composite mononuclear cell defi - of an obvious myelodysplastic syndrome, underscoring the ciency, atypical mycobacterial and viral infection, and progres - fact that immunodeficiency is a cardinal feature of the disor - sion to myelodysplasia and leukemia. Strikingly, a number of der. In the series of 4 subjects described in 2011, one had mild patients also develop pulmonary alveolar proteinosis (PAP). anemia (10.3 g/dL) and one mild thrombocytopenia Patients typically present in their 3 rd or 4 th decade, although his - (123 ¥10 9/L), yet 2 required hematopoietic stem cell transplan - torical blood counts may show pre-existing monocytopenia tation (one for refractory mycobacterial infection, one for PAP) for ten years or more. A substantial number of patients devel - and a third died of influenza H1N1 prior to transplantation. -

PBX3 and MEIS1 Cooperate in Hematopoietic Cells to Drive Acute
Published OnlineFirst January 8, 2016; DOI: 10.1158/0008-5472.CAN-15-1566 Cancer Molecular and Cellular Pathobiology Research PBX3 and MEIS1 Cooperate in Hematopoietic Cells to Drive Acute Myeloid Leukemias Characterized by a Core Transcriptome of the MLL-Rearranged Disease Zejuan Li1, Ping Chen1, Rui Su2, Chao Hu1,2,3, Yuanyuan Li1, Abdel G. Elkahloun4, Zhixiang Zuo1,2, Sandeep Gurbuxani5, Stephen Arnovitz1, Hengyou Weng1,2, Yungui Wang1,2,3, Shenglai Li1, Hao Huang1, Mary Beth Neilly1, Gang Greg Wang6, Xi Jiang1,2, Paul P. Liu4, Jie Jin3, and Jianjun Chen1,2 Abstract Overexpression of HOXA/MEIS1/PBX3 homeobox genes is sion profiling of hematopoietic cells demonstrated that PBX3/ the hallmark of mixed lineage leukemia (MLL)-rearranged MEIS1 overexpression, but not HOXA9/MEIS1, HOXA9/PBX3, acute myeloid leukemia (AML). HOXA9 and MEIS1 are con- or HOXA9 overexpression, recapitulated the MLL-fusion–medi- sideredtobethemostcriticaltargetsofMLLfusionsandtheir ated core transcriptome, particularly upregulation of the endog- coexpression rapidly induces AML. MEIS1 and PBX3 are not enous Hoxa genes. Disruption of the binding between MEIS1 individually able to transform cells and were therefore hypoth- andPBX3diminishedPBX3/MEIS1–mediated cell transforma- esized to function as cofactors of HOXA9. However, in this tion and HOX gene upregulation. Collectively, our studies study, we demonstrate that coexpression of PBX3 and MEIS1 strongly implicate the PBX3/MEIS1 interaction as a driver of (PBX3/MEIS1), without ectopic expression of a HOX gene, is cell transformation and leukemogenesis, and suggest that this sufficient for transformation of normal mouse hematopoietic axis may play a critical role in the regulation of the core stem/progenitor cells in vitro.Moreover,PBX3/MEIS1 overex- transcriptional programs activated in MLL-rearranged and pression also caused AML in vivo, with a leukemic latency HOX-overexpressing AML. -

Letters to the Editor
LETTERS TO THE EDITOR The significant upregulation of Gata1 and EpoR mRNA Mice over-expressing human erythropoietin expression in hEPO over-expressing tg6 mice was con - indicate that erythropoietin enhances expression firmed by analyzing the spleen as a major source of of its receptor via up-regulated Gata1 and Tal1 hematopoiesis (Figure 2A and B). To further dissect the complexity of changes in the transcriptional network, the analysis of Myb mRNA expression served as marker for The development of medullary hematopoiesis is char - adult definitive erythroblasts, 10 showing significantly acterized by a specific expression profile of hematopoiet - ic transcription factors, including GATA transcription fac - tors. At mid-gestation, when hematopoiesis is newly A GATA 1 2 established in the bone marrow of human fetuses, initial - wt ly high GATA2 expression becomes subsequently down- regulated, while GATA1 expression increases in parallel. 1 1.5 Both transcription factors bind to overlapping sets of tg6 hematopoietic downstream target genes, often at distinct 1 sites, to regulate the balance between proliferation and differentiation. Chromatin occupancy by GATA1 and 0.5 GATA2 can change in the course of hematopoietic differ - entiation, leading to the so-called GATA switch. 2 Thus, a 0 n d7 d21 d49 i spatio-temporal regulation of GATA1 or GATA2 activities t c is required within lineage-specific differentiation. During a - erythroid differentiation GATA1 expression peaks at the b B GATA 2 3 o level of colony-forming units (CFU-E), where erythro - t 2 e poietin (Epo) exerts most specifically its effects, but v i t blocks terminal maturation if constitutively over- a 1.5 l 4 e expressed. -

Secondary Pulmonary Alveolar Proteinosis in Hematologic
review Secondary pulmonary alveolar proteinosis in hematologic malignancies Chakra P Chaulagain a,*, Monika Pilichowska b, Laurence Brinckerhoff c, Maher Tabba d, John K Erban e a Taussig Cancer Institute of Cleveland Clinic, Department of Hematology/Oncology, Cleveland Clinic in Weston, FL, USA, b Department of Pathology, Tufts Medical Center Cancer Center & Tufts University School of Medicine, Boston, MA, USA, c Department of Surgery, Tufts Medical Center Cancer Center & Tufts University School of Medicine, Boston, MA, USA, d Division of Critical Care, Pulmonary and Sleep Medicine, Tufts Medical Center Cancer Center & Tufts University School of Medicine, Boston, MA, USA, e Division of Hematology/Oncology, Tufts Medical Center Cancer Center & Tufts University School of Medicine, Boston, MA, USA * Corresponding author at: Cleveland Clinic Florida, 2950 Cleveland Clinic Blvd., Weston, FL 33331, USA. Tel.: +1 954 659 5840; fax: +1 954 659 5810. Æ [email protected] Æ Received for publication 29 January 2014 Æ Accepted for publication 1 September 2014 Hematol Oncol Stem Cell Ther 2014; 7(4): 127–135 ª 2014 King Faisal Specialist Hospital & Research Centre. Published by Elsevier Ltd. All rights reserved. DOI: http://dx.doi.org/10.1016/j.hemonc.2014.09.003 Abstract Pulmonary alveolar proteinosis (PAP), characterized by deposition of intra-alveolar PAS positive protein and lipid rich material, is a rare cause of progressive respiratory failure first described by Rosen et al. in 1958. The intra-alveolar lipoproteinaceous material was subsequently proven to have been derived from pulmonary surfactant in 1980 by Singh et al. Levinson et al. also reported in 1958 the case of 19- year-old female with panmyelosis afflicted with a diffuse pulmonary disease characterized by filling of the alveoli with amorphous material described as ‘‘intra-alveolar coagulum’’. -

Mutational Landscape and Clinical Outcome of Patients with De Novo Acute Myeloid Leukemia and Rearrangements Involving 11Q23/KMT2A
Mutational landscape and clinical outcome of patients with de novo acute myeloid leukemia and rearrangements involving 11q23/KMT2A Marius Billa,1,2, Krzysztof Mrózeka,1,2, Jessica Kohlschmidta,b, Ann-Kathrin Eisfelda,c, Christopher J. Walkera, Deedra Nicoleta,b, Dimitrios Papaioannoua, James S. Blachlya,c, Shelley Orwicka,c, Andrew J. Carrolld, Jonathan E. Kolitze, Bayard L. Powellf, Richard M. Stoneg, Albert de la Chapelleh,i,2, John C. Byrda,c, and Clara D. Bloomfielda,c aThe Ohio State University Comprehensive Cancer Center, Columbus, OH 43210; bAlliance for Clinical Trials in Oncology Statistics and Data Center, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210; cDivision of Hematology, Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210; dDepartment of Genetics, University of Alabama at Birmingham, Birmingham, AL 35294; eNorthwell Health Cancer Institute, Zucker School of Medicine at Hofstra/Northwell, Lake Success, NY 11042; fDepartment of Internal Medicine, Section on Hematology & Oncology, Wake Forest Baptist Comprehensive Cancer Center, Winston-Salem, NC 27157; gDepartment of Medical Oncology, Dana-Farber/Partners Cancer Care, Boston, MA 02215; hHuman Cancer Genetics Program, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210; and iDepartment of Cancer Biology and Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210 Contributed by Albert de la Chapelle, August 28, 2020 (sent for review July 17, 2020; reviewed by Anne Hagemeijer and Stefan Klaus Bohlander) Balanced rearrangements involving the KMT2A gene, located at patterns that include high expression of HOXA genes and thereby 11q23, are among the most frequent chromosome aberrations in contribute to leukemogenesis (14–16).