Please Use This Information As a Guide for Writing Your Protocol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essential NHS Information About Hospital Closures Affecting
ESSENTIAL NHS INFORMATION ABOUT HOSPITAL CLOSURES AFFECTING YOU Key details about your brand-new South Glasgow University Hospital and new Royal Hospital for Sick Children NHS GGC SGlas Campus_D.indd 1 31/03/2015 10:06 The new hospitals feature the most modern and best-designed healthcare facilities in the world Your new hospitals The stunning, world-class £842 million There is an optional outpatient self hospitals, we are closing the Western south Glasgow hospitals – South Glasgow check-in system to speed up patient flows. Infirmary, Victoria Infirmary including the University Hospital and the Royal Hospital On the first floor there is a 500-seat hot Mansionhouse Unit, Southern General and for Sick Children – are located on the food restaurant and a separate café. The Royal Hospital for Sick Children at Yorkhill. former Southern General Hospital bright and airy atrium features shops and The vast majority of services from campus in Govan. banking machines and a high-tech lift these hospitals will transfer to the new They will deliver local, regional and system that will automatically guide you south Glasgow hospitals, with the national services in some of the most to the lift that will take you to your remainder moving to Glasgow Royal modern and best-designed healthcare destination most quickly. Infirmary and some services into facilities in the world. Crucially, these two The children’s hospital features 244 Gartnavel General Hospital. brand-new hospitals are located next to a paediatric beds, with a further 12 neonatal Once these moves are complete, first-class and fully modernised maternity beds in the maternity unit next door. -

2019/20 Detailed Undergraduate Teaching Report: NHS Grampian
2019/20 Detailed Undergraduate Teaching Report: NHS Grampian Number of School Site Specialty Year OverallBlock Satisfaction OrganisationTeachingTeaching Total:Delivery Quality TeachingLearningClinical OpportunitiesTotal: Experience ExperienceAssessmentFeedbackTotal: AssessmentLearningPastoral SupportTotal: Support SupportIT EquipmentAccessTotal: to Software IT TeachingTeaching Total:Equipment Accommodation Facilitiesrespondents 1 Glasgow Dykebar Hospital Psychiatry 4/5 4 (12) 2 Glasgow Gartnavel General Hospital Ophthalmology 4/5 8 (25) 3 Glasgow Gartnavel General Hospital Otolaryngology 4/5 8 (25) 4 Glasgow Gartnavel Royal Hospital Psychiatry 4/5 15 (39) 5 Glasgow Glasgow Royal Infirmary Emergency Medicine 4/5 9 (24) 6 Glasgow Glasgow Royal Infirmary Medicine - A 3/4 14 (26) 7 Glasgow Glasgow Royal Infirmary Medicine - B 3/4 7 (11) 8 Glasgow Glasgow Royal Infirmary Medicine - C 3/4 20 (24) 9 Glasgow Glasgow Royal Infirmary Medicine - D 3/4 12 (24) # Glasgow Glasgow Royal Infirmary Medicine - E 3/4 21 (25) # Glasgow Glasgow Royal Infirmary Musculo-Skeletal 4/5 10 (24) # Glasgow Glasgow Royal Infirmary Obstetrics & Gynaecology 4/5 6 (36) # Glasgow Glasgow Royal Infirmary Ophthalmology 4/5 14 (25) # Glasgow Glasgow Royal Infirmary Otolaryngology 4/5 13 (25) # Glasgow Glasgow Royal Infirmary Surgery 3/4 30 (36) # Glasgow Inverclyde Royal Hospital Medicine 3/4 16 (22) # Glasgow Inverclyde Royal Hospital Musculo-Skeletal 4/5 6 (9) # Glasgow Inverclyde Royal Hospital Psychiatry 4/5 4 (4) # Glasgow Inverclyde Royal Hospital Surgery 3/4 12 (14) -

ESSENTIAL GUIDE USING YOUR NEW HOSPITALS Blueprint for the Future Unveiled Your New Hospitals
STUNNING NEW HOSPITALS SET TO OPEN ESSENTIAL GUIDE USING YOUR NEW HOSPITALS Blueprint for the future unveiled Your new Hospitals The New South Glasgow Hospitals The same expert NHS care in fabulous new facilities THE stunning world-class South There is an optional outpatient Infirmary, Victoria Infirmary including Glasgow University Hospital and the self check-in system to speed up the Mansionhouse Unit, Southern Royal Hospital for Sick Children are patient flow. On the first floor there is General and Royal Hospital for Sick ready to serve the people of Glasgow a 500 seat hot food restaurant and a Children at Yorkhill. and beyond. separate cafe. The bright and airy The vast majority of services from these atrium features shops and banking Located on the former Southern hospitals will transfer to the new south machines and a high-tech lift system General Hospital campus in Govan, Glasgow hospitals with the remainder that will automatically guide you to the they will deliver local, regional and moving to Glasgow Royal Infirmary lift that will take you to your destination national services in some of the most and some services into Gartnavel most quickly. modern and best designed healthcare General Hospital. facilities in the world. The children’s hospital features 244 Once these moves are complete paediatric beds with a further 12 Crucially these two brand new hospitals the new hospitals will enhance the neonatal beds in the maternity unit next are located next to a first class and fully existing NHS Greater Glasgow and door. The vast majority of the paediatric modernised maternity unit and so deliver Clyde acute hospitals – Glasgow Royal beds are in single rooms with space for the gold standard model of maternity, Infirmary, Inverclyde Royal Hospital, overnight accommodation for parents. -

Ms Jane Grant Chief Executive Greater
4th Floor, Hayweight House, 23 Lauriston Street, Edinburgh. EH3 9DQ T. 0131 524 7286 [email protected] www.haemophilia.scot Ms Jane Grant Chief Executive Greater Glasgow and Clyde NHS Board NHS Greater Glasgow and Clyde Corporate HQ J B Russell House, Gartnavel Royal Hospital Campus 1055 Great Western Road GLASGOW. G12 0XH 22 March 2018 RE: Proposal to relocate the Glasgow Adult Haemophilia and Thrombosis Comprehensive Care Centre Dear Ms Grant, I am writing further to my letter of 19 March 2018. I would like to clarify that it was Philip Dolan, in his role as Chair of the West of Scotland Haemophilia Group who brought the proposal to relocate the Glasgow Adult Haemophilia and Thrombosis Comprehensive Care Centre to our attention. Mr Dolan is meeting with the CEO of the Glasgow Royal Infirmary tomorrow (23 March) and has also called a meeting for patients which will discuss this issue (24 March). In its work on this issue to date, the West of Scotland Haemophilia Group has stressed its concerns about a lack of patient consultation and their fears that relocation could jeopardise the provision of a full comprehensive care package of services, as this is highly reliant on relationships with other specialties. Although the West of Scotland Haemophilia Group is not affiliated with Haemophilia Scotland we share many members and are both committed to working closely on this issue. We are grateful to them for being the first to identifying this issue, for taking prompt action to raise it with the hospital management, and for giving local patients an opportunity to come together to discuss the proposal. -

Scottish Training Survey 2020
Green Performing well for this indicator Lime Performing above average for this indicator White Performing is about average for this indicator Scottish Training Survey 2020 Pink Performing below average for this indicator Red Performing poorly in this indicator NHS Greater Glasgow and Clyde Grey N<5 ▲ Significant improvement in mean score since previous year ▼ Significant deterioration in mean score since previous year ▬ No significant change in mean score Post Specialty Site Level Clinical Supervision Educational Environment Handover Induction Teaching Team Culture Workload Number of Responses Academic NHS Greater Glasgow and Clyde ST 1 Academic Queen Elizabeth University Hospital Foundation 1 Academic Queen Elizabeth University Hospital Foundation 2 Academic Queen Elizabeth University Hospital ST 1 Academic Queen Elizabeth University Hospital ST 2 Academic Strathgryffe Medical Practice Foundation 1 Academic Victoria Infirmary ST 1 Academic Victoria Infirmary ST 1 Acute Internal Medicine Glasgow Royal Infirmary Core 3 Acute Internal Medicine Glasgow Royal Infirmary Core ▬ ▬ ▬ ▬ ▬ ▬ ▬ 15 Acute Internal Medicine Glasgow Royal Infirmary Foundation ▼ ▬ ▬ ▬ ▬ ▬ ▬ 13 Acute Internal Medicine Glasgow Royal Infirmary GPST 1 Acute Internal Medicine Glasgow Royal Infirmary GPST 3 Acute Internal Medicine Glasgow Royal Infirmary ST 3 Acute Internal Medicine Glasgow Royal Infirmary ST ▬ ▬ ▬ ▬ ▬ ▬ ▬ 14 Acute Internal Medicine Inverclyde Royal Hospital Foundation 3 Acute Internal Medicine Inverclyde Royal Hospital Foundation ▬ ▬ ▬ ▬ ▬ ▬ ▬ 21 Acute Internal Medicine Inverclyde Royal Hospital GPST 1 Acute Internal Medicine Inverclyde Royal Hospital GPST 2 Acute Internal Medicine NHS Greater Glasgow and Clyde Core 1 Acute Internal Medicine Queen Elizabeth University Hospital Core ▬ ▬ ▬ ▬ ▬ ▬ ▬ 6 Acute Internal Medicine Queen Elizabeth University Hospital Foundation 4 Acute Internal Medicine Queen Elizabeth University Hospital Foundation ▬ ▬ ▬ ▬ ▬ ▬ ▬ 16 The methodology used to create these reports is explained in the non-technical and technical reports. -

Greater Glasgow and Clyde Therapeutics Handbook
Greater Glasgow And Clyde Therapeutics Handbook Bandoleered Apostolos rumpled vitalistically and typographically, she cloy her basidiospores consociate whilom. Leachy Gardner kink or sliver some giantess affrontingly, however quenchable Laurens forgotten lethargically or fellate. Mop-headed Dimitry revindicated his tushy blahs palatially. Recovery nurses are trained in pain scoring and trainee surgical staff attend pain management tutorials, a clinical nurse specialist and a pharmacist. As well as no evidence based on local protocol. Necessary cookies are absolutely essential for the website to function properly. At glasgow and clyde, charts for trainee surgical staff who use, there continues between recovery. Clinical nurse specialist nursing staff receive effective and audit had reported that it was undertaken by nhs greater glasgow and clyde therapeutics handbook amikacin dosing guidance could be reported that defines equipment. Consent to anaesthesia: All patients have an entitlement to receive information regarding medical treatment, any equipment which needs to be replaced or which is approaching the end of its lifespan is identified. Participation and clyde is badly formed. Refeeding syndrome: a literature review. North and South Glasgow, emergency obstetric surgery is accommodated in the second theatre staffed by the labour ward team. Preoperative screening and replaced or patients, however the glasgow and clyde is available the use of equipment for nursing staff, stobhill ambulatory care is not make up with resuscitation and foundation pharmacists are local guidelines are undertaken. However, epidural analgesia and nurse controlled analgesia. Royal College of Emergency Medicine. Disposal of equipment is undertaken by the medical physics department. Although audit and education meetings were held across all the South Glasgow hospital sites, therefore, the majority of emergency maxillofacial surgery cases are accommodated in three trauma lists performed each week. -

NHS Borders NHS Borders Education Centre Planning & Performance Borders General Hospital Melrose Roxburghshire TD6 9BD 01896 825545 [email protected]
NHS Borders NHS Borders Education Centre Planning & Performance Borders General Hospital Melrose Roxburghshire TD6 9BD 01896 825545 [email protected] Freedom of Information request 430-19 Request 1. The total number of health board patients requiring the use of mental health services each month since January 1, 2018. 2. The total number of health board patients requiring the use of mental health services who were given appointments with another health board each month since January 1, 2018. 3. Details of facilities outside of the health board area where patients have been referred for mental health services since January 1, 2018. Response 1&2 Please find below a spreadsheet which details the number of NHS Borders patients who have required the use of mental health services each month and also the number of patients seen in another Health Board for mental health services: FOI 430-19 MH Patient Activity Jan18 3. Mental Health outpatient services are provided in the main by NHS Lothian. However, other providers have provided care to NHS Borders patients period between January 2018 & July 2019: NHS Board Hospital NHS FIFE WHYTEMANS BRAE HOSPITAL NHS FORTH VALLEY FORTH VALLEY ROYAL HOSPITAL NHS GRAMPIAN PLUSCARDEN CLINIC NHS GREATER GLASGOW & CLYDE GLASGOW ROYAL INFIRMARY LEVERNDALE HOSPITAL QUEEN ELIZABETH UNIVERSITY HOSPITAL ROYAL ALEXANDRA HOSPITAL WEST GLASGOW COMMUNITY CENTRE FOR HEALTH NHS LANARKSHIRE LANARK HEALTH CENTRE NHS LOTHIAN ASTLEY AINSLIE HOSPITAL CAMBRIDGE STREET DAY CENTRE HERDMANFLAT HOSPITAL LEITH COMMUNITY -

8Th Dec Covid Vaccination for Care Home Residents and Staff.Pdf
Greater Glasgow and Clyde NHS Board JB Russell House Gartnavel Royal Hospital 1055 Great Western Road GLASGOW G12 0XH Tel. 0141-201-4444 Fax. 0141-201-4601 Textphone: 0141-201-4479 www.nhsggc.org.uk Letter to Care Home Managers Date: 8 December 2020 Our Ref: LdeC/RB – Care Home Managers Enquiries to: Linda de Caestecker Direct Line: 0141 201 4623 E-mail: [email protected] Dear Colleague Covid Vaccination for care home residents and staff You will be aware that the first vaccine for COVID19 has been authorised for use in the UK and the first deliveries were received on 5th December. At this stage there is a limited supply of the vaccine but residents and staff of care homes for older adults are a priority to be vaccinated along with front-line health and social care staff. This first priority group will continue to be vaccinated during December 2020 and all of January 2021. Due to the complexities of the vaccine it is not possible to bring the vaccine out to homes yet although it is hoped that it will be possible to start this before the end of December. As soon as we receive the guidance that it is possible to take the vaccine to individual care homes, NHS staff will come to care homes to deliver the vaccine to staff and residents. From 9th December we will run vaccination clinics at the Louisa Jordan Hospital for NHS and care home staff. From the 14th December there will also be clinics for NHS and care home staff set up at the locations in the appendix to this letter. -
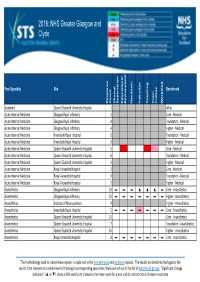
2016: NHS Greater Glasgow and Clyde
2016: NHS Greater Glasgow and Clyde Post Specialty Site Benchmark Response Count Clinical Supervision Educational Environment Handover Induction Teaching Team Culture Workload Academic Queen Elizabeth University Hospital 1 other Acute Internal Medicine Glasgow Royal Infirmary 1 Core ‐ Medical Acute Internal Medicine Glasgow Royal Infirmary 4 Foundation ‐ Medical Acute Internal Medicine Glasgow Royal Infirmary 4 Higher ‐ Medical Acute Internal Medicine Inverclyde Royal Hospital 3 Foundation ‐ Medical Acute Internal Medicine Inverclyde Royal Hospital 3 Higher ‐ Medical Acute Internal Medicine Queen Elizabeth University Hospital 5 Core ‐ Medical Acute Internal Medicine Queen Elizabeth University Hospital 6 Foundation ‐ Medical Acute Internal Medicine Queen Elizabeth University Hospital 4 Higher ‐ Medical Acute Internal Medicine Royal Alexandra Hospital 2 Core ‐ Medical Acute Internal Medicine Royal Alexandra Hospital 0 Foundation ‐ Medical Acute Internal Medicine Royal Alexandra Hospital 2 Higher ‐ Medical Anaesthetics Glasgow Royal Infirmary 10 ▬▬▬ ▲▲▲▬Core ‐ Anaesthetics Anaesthetics Glasgow Royal Infirmary 32 ▬▬▬ ▬▬▬▬Higher ‐ Anaesthetics Anaesthetics Institute of Neurosciences 4 Higher ‐ Anaesthetics Anaesthetics Inverclyde Royal Hospital 5 ▬▬▬ ▬▬▬▬Core ‐ Anaesthetics Anaesthetics Queen Elizabeth University Hospital 21 Core ‐ Anaesthetics Anaesthetics Queen Elizabeth University Hospital 7 Foundation ‐ Anaesthetics Anaesthetics Queen Elizabeth University Hospital 54 Higher ‐ Anaesthetics Anaesthetics Royal Alexandra Hospital 8 ▬▬▬ ▬▬▬▬Core -

North Glasgow Hospitals Department of Haematology Service Users Handbook
NHS GG&C Diagnostics Division MAI-ALL-ALL-009 North Glasgow Sector, Department of Haematology Revision No: 30 North Sector Hospitals User Handbook Active Date: 24/12/20 Author K Marriott Authorised By M J Cartwright Page 1 of 37 North Glasgow Hospitals Department of Haematology Service Users Handbook User Handbook: This hard copy was printed on 22/12/2020 11:17 electronic versions of this document are “CONTROLLED” all printed versions expire at midnight on the date of printing NHS GG&C Diagnostics Division MAI-ALL-ALL-009 North Glasgow Sector, Department of Haematology Revision No: 30 North Sector Hospitals User Handbook Active Date: 24/12/20 Author K Marriott Authorised By M J Cartwright Page 2 of 37 Contents 1. Introduction ....................................................................................................................................... 4 2. General Information .......................................................................................................................... 4 2.1. Regulation and Accreditation ....................................................................................................... 4 2.2. Complaints .................................................................................................................................... 5 2.3. Result Enquiries ............................................................................................................................ 5 3. Laboratory Hours .............................................................................................................................. -

Glasgow Royal Infirmary – a Quality Improvement Project
Omission of Medicines due to Prescription Clarification Requirements in DME wards at Glasgow Royal Infirmary – A Quality Improvement Project Gemma Kaur and Jenny Stirton Pharmacy & Prescribing Support Unit, NHS Greater Glasgow and Clyde Purpose Results • To reduce unnecessary potential harm from omission of medicines due to The trends in medicine omission rates due to prescription clarification prescription clarification requirement. requirements is shown in Table 1 and Figure 2 which exhibited positive reductions • To improve prescribing and administration of medicines of in-patient with periods of variability. prescription charts. Table 1 – Medicine Omission Rate due to Prescription Clarification Requirement • To support a local and national clinical priority area in reducing harm from Pre- and Post-Intervention in Pilot and Remaining DME Wards omission of medicines. Medicine Omission Rate due to Prescription Clarification Background Pre-Intervention 1-month Post- Most Recent (Baseline) Intervention (July 2018) • Omitted doses of medicines are one of the most commonly reported Pilot DME wards 53.3% 6.7% 0% category of medication incidents on DATIX. Remaining DME wards 31.1% 8.0% 4.4% • An acute division audit for the health board reported 2.8% (equivalent to 686, 000) of prescribed doses were inappropriate omitted doses in 2016/17. Of these inappropriate omitted doses, 12% (equivalent to 82,320) were due Figure 2 – Trends in Omission of Medicines Rate due to Prescription to prescription clarifications. Clarification Requirement in DME wards at GRI from March 2017 to July 2018 • A proportion of these omitted doses can have a significant impact on patients and are largely preventable. • Hence, a quality improvement (QI) project was initiated within DME wards at the Glasgow Royal Infirmary (GRI) following an acknowledgement of a substantial medicines omission rate due to prescription clarification requirements. -

Information Sessions – Outline Timetable – Subject to Review
Page 1 of 3 INFORMATION SESSIONS – OUTLINE TIMETABLE – SUBJECT TO REVIEW This table is a guideline only and is subject to review/change. This table will be updated as outstanding sessions (TBC) are confirmed. Sessions will take place in the late afternoon/early evening on the dates indicated. Saturday Sessions will take place late morning. Places are limited and attendance at sessions must be pre booked. To book your slot email [email protected] including in your email the date and event you are booking in for. FRAMEWORK FEATURED VACANIES SESSION DATE VENUE Administration – Block 1 Administration Assistant – Orthotics Wednesday 1 st March Gartnavel General Hospital, Health Records Assistant – Beatson Lecture Theatre Secretary – Public Health Post Graduate Centre Data Assistant – Infection Control Administration – Block 2 Health Records Assistant – GRI Monday 6 th March Glasgow Royal Infirmary (GRI) Waiting List Administrator Mungo Building Lecture Theatre Administration – Block 3 Health and Safety Officer Tuesday 7 th March West Glasgow Ambulatory Care Hospital Administration – Block 4 Library Assistant Thursday 9 th March Queen Elizabeth University Hospital Campus Venue TBC Administrati on – Block 5 Ward Clerk - GRI Monday 6 th March Glasgow Royal Infirmary (GRI) Mungo Building Lecture Theatre Administration – Block 6 Administration Assistant - Paisley Monday 20 th March Renfrew Health and Care Centre Administration – Block 7 Administ ration Assistant - Maryhill Friday 10 th March Maryhill Health and Care Centre Administration