Understanding Basic Biology of Mammalian Reproduction: Growth, Development and Mother's Milk by Gary Kwiecinski University Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caesarean Section Or Vaginal Delivery in the 21St Century
CAESAREAN SECTION OR VAGINAL DELIVERY IN THE 21ST CENTURY ntil the 20th Century, caesarean fluid embolism. The absolute risk of trans-placentally to the foetus, prepar- section (C/S) was a feared op- death with C/S in high and middle- ing the foetus to adopt its mother’s Ueration. The ubiquitous classical resource settings is between 1/2000 and microbiome. C/S interferes with neonatal uterine incision meant high maternal 1/4000 (2, 3). In subsequent pregnancies, exposure to maternal vaginal and skin mortality from bleeding and future the risk of placenta previa, placenta flora, leading to colonization with other uterine rupture. Even with aseptic surgi- accreta and uterine rupture is increased. environmental microbes and an altered cal technique, sepsis was common and These conditions increase maternal microbiome. Routine antibiotic exposure lethal without antibiotics. The operation mortality and severe maternal morbid- with C/S likely alters this further. was used almost solely to save the life of ity cumulatively with each subsequent Microbial exposure and the stress of a mother in whom vaginal delivery was C/S. This is of particular importance to labour also lead to marked activation extremely dangerous, such as one with women having large families. of immune system markers in the cord placenta previa. Foetal death and the use blood of neonates born vaginally or by of intrauterine foetal destructive proce- Maternal Benefits C/S after labour. These changes are absent dures, which carry their own morbidity, C/S has a modest protective effect against in the cord blood of neonates born by were often preferable to C/S. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Physiological Substrates of Mammalian Monogamy: the Prairie Vole Model
Neuroscienceand BiobchavioralReviews, Vol. 19, No. 2, pp. 303-314, 1995 Copyright© 1995 ElsevierScience Lid Pergamon Printed in the USA. All rightsreserved 0149-7634/95 $9.50 + .00 0149-7634(94)00070-0 Physiological Substrates of Mammalian Monogamy: The Prairie Vole Model C. SUE CARTER, .1 A. COURTNEY DEVRIES,* AND LOWELL L. GETZt *DeFartment of Zoology, University of Maryland, College Park, MD 20742, and tDepartment of Ecology, Ethology, and Evolution, University of Illinois, Urbana, IL 61801 CARTER, C. S., A. C. DEVRIES AND L. L. GETZ. Physiological substrates of mammalian monogamy: The prairie vole model. NEUROSCI BIOBEHAV REV 19(2) 303-314, 1995.-Prairie voles (Microtus ochrogaster) are described here as a model system in which it is possible to examine, within the context of natural history, the proximate processes regulating the social and reproductive behaviors that characterize a monogamous social system. Neuropeptides, including oxytocin and vasopressin, and tihe adrenal glucocorticoid, corticosterone, have been implicated in the neural regulation of partner prefer- ences, and in the male, vasopressin has been implicated in the induction of selective aggression toward strangers. We hypothesize here that interactions among oxytocin, vasopressin and glucocorticoids could provide substrates for dynamic changes in social and agonistic behaviors, including those required in the development and expression of monogamy. Results from research with voles suggest that the behaviors characteristics of monogamy, including social attachments and biparental care, may be modified by hormones during development and may be regulated by different mechanisms in males and females. Prairie voles Social behavior Attachment Monogamy Oxytocin Vasopressin Adrenal steroids Corticosterone Sex differences MONOGAMY IN MAMMALS viduals within a family group (that remain with the family as "helpers"). -

Vocabulary: Sharks
Grades 11-12 - Vocabulary: Sharks Dermal Denticles – Tiny tooth-shaped scales that cover a shark’s body. Dermal Denticles have the same structure as teeth - enamel, dentine, pulp, epidermis, and dermis. Counter Shading - Having a dark dorsal or upper side and a lighter colored underside. Lateral Line – A row of sensors used by sharks and other fish, which detect vibrations. Cartilage – The material that makes up a shark’s skeleton (not bone), and is also found in our ears and nose. Basihyal - A sharks tongue, composed of a small piece of cartilage on the bottom of a sharks’ mouth. Carnivore - An animal that eats meat. Megalodon - An ancient shark that lived between 5 and 1.6 million years ago. Serrated Tooth - A tooth with a jagged edge that is used for sawing. Dorsal Fin - Primary fin located on the back of fishes and certain marine mammals. Pectoral Fins - Either of the anterior pairs of fins. Barbels - Sensory projections near the nostrils and mouth of some sharks, i.e. nurse sharks. They are whisker-like feelers used to taste and feel. Gills - Respiratory organs that fish use to absorb oxygen from the water in order to breathe. Snout - The tip of a shark’s head. Pup - A newly born or hatched shark. Claspers - Two finger like projections on the rear underside of male sharks. Ampullae of Lorenzini - Pores scattered about the head of sharks that are filled with a jellylike substance that can sense temperature change and weak electrical impulses given off by sick prey. Fusiform – A streamlined, oval shape body. -

Female and Male Gametogenesis 3 Nina Desai , Jennifer Ludgin , Rakesh Sharma , Raj Kumar Anirudh , and Ashok Agarwal
Female and Male Gametogenesis 3 Nina Desai , Jennifer Ludgin , Rakesh Sharma , Raj Kumar Anirudh , and Ashok Agarwal intimately part of the endocrine responsibility of the ovary. Introduction If there are no gametes, then hormone production is drastically curtailed. Depletion of oocytes implies depletion of the major Oogenesis is an area that has long been of interest in medicine, hormones of the ovary. In the male this is not the case. as well as biology, economics, sociology, and public policy. Androgen production will proceed normally without a single Almost four centuries ago, the English physician William spermatozoa in the testes. Harvey (1578–1657) wrote ex ovo omnia —“all that is alive This chapter presents basic aspects of human ovarian comes from the egg.” follicle growth, oogenesis, and some of the regulatory mech- During a women’s reproductive life span only 300–400 of anisms involved [ 1 ] , as well as some of the basic structural the nearly 1–2 million oocytes present in her ovaries at birth morphology of the testes and the process of development to are ovulated. The process of oogenesis begins with migra- obtain mature spermatozoa. tory primordial germ cells (PGCs). It results in the produc- tion of meiotically competent oocytes containing the correct genetic material, proteins, mRNA transcripts, and organ- Structure of the Ovary elles that are necessary to create a viable embryo. This is a tightly controlled process involving not only ovarian para- The ovary, which contains the germ cells, is the main repro- crine factors but also signaling from gonadotropins secreted ductive organ in the female. -
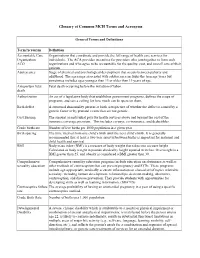
Glossary of Common MCH Terms and Acronyms
Glossary of Common MCH Terms and Acronyms General Terms and Definitions Term/Acronym Definition Accountable Care Organizations that coordinate and provide the full range of health care services for Organization individuals. The ACA provides incentives for providers who join together to form such ACO organizations and who agree to be accountable for the quality, cost, and overall care of their patients. Adolescence Stage of physical and psychological development that occurs between puberty and adulthood. The age range associated with adolescence includes the teen age years but sometimes includes ages younger than 13 or older than 19 years of age. Antepartum fetal Fetal death occurring before the initiation of labor. death Authorization An act of a legislative body that establishes government programs, defines the scope of programs, and sets a ceiling for how much can be spent on them. Birth defect A structural abnormality present at birth, irrespective of whether the defect is caused by a genetic factor or by prenatal events that are not genetic. Cost Sharing The amount an individual pays for health services above and beyond the cost of the insurance coverage premium. This includes co-pays, co-insurance, and deductibles. Crude birth rate Number of live births per 1000 population in a given year. Birth spacing The time interval from one child’s birth until the next child’s birth. It is generally recommended that at least a two-year interval between births is important for maternal and child health and survival. BMI Body mass index (BMI) is a measure of body weight that takes into account height. -

13. Van Dyke, J.U. 2014. Cues for Reproduction In
Cues for Reproduction in Squamate Reptiles 109 CHAPTER 5 Cues for Reproduction in Squamate Reptiles James U. Van Dyke 5.1 INTRODUCTION To maximize fitness, animals should initiate reproduction based on information from suites of cues that communicate three variables critical to reproductive success: 1) environmental conduciveness for successful reproduction, and survival of offspring and (usually) parents; 2) physiological capability of parents to reproduce; and 3) likelihood of successful mating. Squamates vary widely in reproductive mode (egg-laying, or oviparity vs. live birth, or viviparity), reproductive frequency (including reproducing only once, i.e., semelparity), and output (Tinkle et al. 1970; Dunham et al. 1988), all of which may alter the phenology of gametogenesis and embryonic development relative to season, physiological state (i.e., body condition), courtship, and mating. These phenomenological differences necessitate divergent reproductive decision-making approaches that may be informed by different suites of cues. In addition, specifi c components of reproduction, including gametogenesis and mating behavior, may not be stimulated by the same environmental or physiological cues. The purpose of this review is to discuss the current state of knowledge of the mechanisms squamates use as cues for the decision to reproduce. Here, the decision to reproduce is defi ned as analogous to a life-history allocation decision (e.g., Dunham et al. 1989), rather than as a result of conscious thought processes. The endocrine connections of the School of Biological Sciences, Heydon-Laurence Bldg A08, University of Sydney, New South Wales, 2006, Australia. 110 Reproductive Biology and Phylogeny of Lizards and Tuatara hypothalamic-pituitary-gonadal axis are briefl y reviewed because they are critical to communicating information from reproductive cues to the brain, gonads, and accessory reproductive organs. -

Onychophorology, the Study of Velvet Worms
Uniciencia Vol. 35(1), pp. 210-230, January-June, 2021 DOI: http://dx.doi.org/10.15359/ru.35-1.13 www.revistas.una.ac.cr/uniciencia E-ISSN: 2215-3470 [email protected] CC: BY-NC-ND Onychophorology, the study of velvet worms, historical trends, landmarks, and researchers from 1826 to 2020 (a literature review) Onicoforología, el estudio de los gusanos de terciopelo, tendencias históricas, hitos e investigadores de 1826 a 2020 (Revisión de la Literatura) Onicoforologia, o estudo dos vermes aveludados, tendências históricas, marcos e pesquisadores de 1826 a 2020 (Revisão da Literatura) Julián Monge-Nájera1 Received: Mar/25/2020 • Accepted: May/18/2020 • Published: Jan/31/2021 Abstract Velvet worms, also known as peripatus or onychophorans, are a phylum of evolutionary importance that has survived all mass extinctions since the Cambrian period. They capture prey with an adhesive net that is formed in a fraction of a second. The first naturalist to formally describe them was Lansdown Guilding (1797-1831), a British priest from the Caribbean island of Saint Vincent. His life is as little known as the history of the field he initiated, Onychophorology. This is the first general history of Onychophorology, which has been divided into half-century periods. The beginning, 1826-1879, was characterized by studies from former students of famous naturalists like Cuvier and von Baer. This generation included Milne-Edwards and Blanchard, and studies were done mostly in France, Britain, and Germany. In the 1880-1929 period, research was concentrated on anatomy, behavior, biogeography, and ecology; and it is in this period when Bouvier published his mammoth monograph. -

A B C Pregnancy Terms and Definitions
Pregnancy Terms and Definitions Obstetrics & Gynecology A After pains or afterbirth pains: Contractions of the uterus that occur after your baby is born, as the uterus returns to its normal size. This may cause cramping for a few days, especially if this is not your first baby or if you are nursing. Amniocentesis: the removal of a sample of amniotic fluid by means of a needle inserted through the mother’s abdominal wall; used for genetic and biochemical analysis of the baby. Amniotic fluid: the liquid surrounding and protecting the baby within the amniotic sac throughout pregnancy. Amniotic sac: the membrane within the uterus that contains the baby and the amniotic fluid. Analgesic: Medication that relieves or reduces pain. Anesthesia: Loss of feeling. There are three ways of doing this: general, local and epidural. Anesthesiologist: A doctor who specializes in the use of anesthesia. Anesthetist: A registered nurse who has special training in anesthesia. Apgar score rating: A system to evaluate the health of your baby immediately after birth. The score can be zero to 10, based on appearance and color, pulse, reflexes, activity and respiration. B Baby blues: A mild depression many women feel in the first few weeks after birth. Braxton-Hicks contractions: Mild, usually painless contractions that occur during the entire pregnancy, but are only felt from the 5th month on. Breech birth: Baby is born feet or buttocks first. C Cephalopelvic disproprition (CPD): Baby’s head is too large for the mother’s pelvic bones. Cervix: the neck of the uterus; Pap smears are taken from the cervix. -

Post-Partum Hysterectomy (Removal of the Uterus/Womb After Giving Birth)
Post-partum hysterectomy (removal of the uterus/womb after giving birth) This leaflet explains what happens when a woman needs a post-partum hysterectomy following complications during giving birth. It explains why and how it is done, and what to expect afterwards. If there is anything you do not understand or if you have any questions, please speak to your midwife or doctor. What is post-partum hysterectomy? This is an operation that involves removal of the uterus (womb). This is an uncommon situation in the UK, with around 1 in 1000 women having this procedure done shortly after childbirth in this hospital, as there is a range of treatments used before such surgery which can save both future fertility and the mother’s life. It may be performed in an emergency to save the life of a woman with persistent bleeding after childbirth. Less frequently, it can be a planned procedure, often at the same time as Caesarean birth. Why is it performed? The most common reason is severe bleeding from the uterus that cannot be controlled by other measures. There is a link to Caesarean birth, particularly if the placenta for the most recent baby is both low in the uterus (placenta praevia), and deeply adherent (placenta grows too deeply into the uterine wall, known as placenta percreta or increta), so not separating fully after the birth of the baby. A more common cause of heavy bleeding is ‘uterine atony’, which is the inability of a womb to contract after the birth, as well as large or multiple fibroids and blood clotting problems. -

Correlates of Reproductive Success in a Population of Nine-Banded Armadillos
Color profile: Disabled Composite Default screen 1815 Correlates of reproductive success in a population of nine-banded armadillos W.J. Loughry, Paulo A. Prodöhl, Colleen M. McDonough, W.S. Nelson, and John C. Avise Abstract: We used microsatellite DNA markers to identify the putative parents of 69 litters of nine-banded armadillos (Dasypus novemcinctus) over 4 years. Male and female parents did not differ in any measure of body size in comparisons with nonparents. However, males observed paired with a female were significantly larger than unpaired males, although paired females were the same size as unpaired females. Females categorized as possibly lactating were significantly larger than females that were either definitely lactating or definitely not lactating. There was no evidence of assortative mating: body-size measurements of mothers were not significantly correlated with those of fathers. Nine- banded armadillos give birth to litters of genetically identical quadruplets. Mothers (but not fathers) of female litters were significantly larger than mothers of male litters, and maternal (but not paternal) body size was positively correlated with the number of surviving young within years, but not cumulatively. There were no differences in dates of birth between male and female litters, nor were there any significant relationships between birth date and maternal body size. Body size of either parent was not correlated with the body sizes of their offspring. Cumulative and yearly reproductive success did not differ between reproductively successful males and females. Average reproductive success (which included apparently unsuccessful individuals) also did not differ between males and females. The majority of adults in the population apparently failed to produce any surviving offspring, and even those that did usually did so in only 1 of the 4 years. -

Modzelewski Et Al (2015)
© 2015. Published by The Company of Biologists Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed. Dgcr8 and Dicer are essential for sex chromosome integrity in male meiosis Andrew J. Modzelewski1¶, Stephanie Hilz2¶, Elizabeth A. Crate1, Caterina T. H. Schweidenback2, Elizabeth A. Fogarty2, Jennifer K. Grenier1, 2, Raimundo Freire3, Paula E. Cohen1* and Andrew Grimson2* Departments of 1Biomedical Sciences and 2Molecular Biology and Genetics, Cornell University, Ithaca, NY 14853 3Unidad de Investigacion, Hospital Universitario de Canarias, Ofra s/n, La Cuesta, La Laguna Tenerife 38320, Spain ¶These authors contributed equally to this manuscript *Corresponding Authors: [email protected]; [email protected] Journal of Cell Science Accepted manuscript JCS Advance Online Article. Posted on 1 May 2015 ABSTRACT Small RNAs play crucial roles in regulating gene expression during mammalian meiosis. To investigate the function of microRNAs and small-interfering RNAs in male meiosis, we generated germ cell-specific conditional deletions of Dgcr8 and Dicer in mice. Analysis of spermatocytes from both conditional knockout lines reveals frequent chromosomal fusions during meiosis, always involving one or both sex chromosomes. RNA sequencing indicates upregulation of Atm in spermatocytes from microRNA-deficient mice, and immunofluorescence imaging demonstrates an increased abundance of activated ATM kinase and mislocalization of phosphoMDC1, an ATM phosphorylation substrate. The Atm 3′UTR contains many potential microRNA target sites; notably, target sites for several miRNAs depleted in both conditional knockout mice are highly effective at promoting repression.