Assessment of Fish and Crab Responses to Human Alteration in Barnegat Bay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUMMER CAMPS Bprcamps.Org • 2021
Boulder Parks & Recreation SUMMER CAMPS BPRcamps.org • 2021 REGISTER TODAY! - Camps fill up fast! Choose from 125+ camp sessions for youth ages 4-17! RESERVOIR CAMPS • GOATS & GARDENS • KIDZ KAMP • GYMNASTICS • DRAMA • POTTERY • SPORTS LEGO ENGINEERING • SAILING • BIKING & MORE! Camp Planner Registration: 303-413-7270 or BPRcamps.org, unless otherwise noted. Page # Page Camp Day/Time Ages # of Days Fee (R) = Resident (N) = Non-Resident May 31-June 4 31-June May June 7-11 June 14-18 June 21-25 2 June28-July July 5-9 July 12-16 July 19-23 July 26-30 2-6 Aug. 9-13 Aug. REZ CAMP provided by the City of Boulder 4 Rez Camp M-Th, 8:30am-4pm 7-14 4 $316(R)/$352(N) • • • • • • • GOATS & GARDENS provided through the City of Boulder 5 Goats & Gardens M-F, 8:30am-3:30pm 5-11 5 $350(R)/$385(N) • • • • • • • • • KIDZ KAMP provided by the City of Boulder 6 Kidz Kamp East M-F, 8:30am-4pm 5-11 5 $567-$700 (2wks) • • • • • GYMNASTICS provided by the City of Boulder 7 Summer Recreational Gymnastics M-F, 9am-12pm 5+ 5 $216(R)/$268(N) • 7 Summer Recreational Gymnastics M/W/F, 9am-10:30am 3-4 3 $65(R)/$81(N) • DANCE provided by our partner Kinesis Dance 7 Dancing Days M-Th, 9am-12pm 3 4 $175 • • 7 All About Dance Camp M-Th, 9am-1 & 1-5pm 6-10 4 $225 • • DRAMA CAMP provided by our partner Miss Joanie at MissJoanieDrama.com 8 Drama M-F, 8:30am-4:30pm 4-11 4-5 $280-$350 • • • • • • • • • • • POTTERY CAMPS provided by our partner Studio Arts Boulder 9 Wheel-throwing M-F, 9-12pm or M-F, 1-4pm 8-11 5 $240 • • • • • • • 9 Wheel-throwing M-F, 9-12pm or M-F, 1-4pm -

2016 Parent Handbook
Sagamore Junior Sailing Program 2016 PARENT HANDBOOK Revised June 10, 2016 Sagamore Junior Sailing Program / LuHi Parent Handbook LuHi, welcome to the 2016 Sagamore Junior Sailing Program! These pages are meant to be a parent’s and future junior sailor’s guide in navigating the child’s way through the rewarding sport of junior sailing. In these pages you will find details on recommended equipment for the children to bring to the program, goals the program will focus for your child and the expectations of the parents themselves. The LuHi Summer Sailing Program is for Grades 4 thru 9. Sagamore Junior Sailing Mission The Sagamore Junior Sailing (SJS) Fleet’s Mission is to provide a fun and educational program for youth interested in sailing and racing. We seek to impart a love of sailing as a life sport while providing the fundamental skills necessary for participants to advance in the sport of sailing as far as their desire, skill and hard work may take them. Our instructors shall provide a curriculum with the most current and effective techniques to create skilled and confident youths who will respect others, care for their equipment and be willing to help fellow sailors on and off the water. Our sailors will be expected to conduct themselves in the Corinthian Spirit during practice and in competition. All Junior Fleet sailors, whether their interest be in cruising or racing, should emerge with an enhanced sense of self-reliance, knowledge of seamanship and a solid foundation in the sport of sailing. An Overview of Junior Sailing Junior Sailing can be like any other full time sport such as baseball, soccer or tennis – what a child puts into it will determine what a child will get out of the program. -
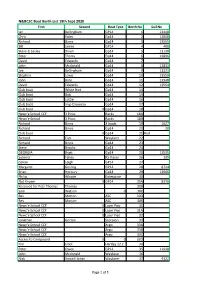
N&BCSC Boat Berth List 19Th Sept 2020
N&BCSC Boat Berth List 19th Sept 2020 First Second Boat Type Berth No Sail No Ian Bellingham GP14 1 13440 Chris Yates Gp14 2 13868 Richard Binns Gp14 3 13555 Bill James GP14 4 408 Steve & Lesley Dixon Gp14 5 13138 Peter Thoms Gp14 6 13896 David Edwards Gp14 7 John Mcdonald Gp14 8 12831 Joe Bellingham Gp14 9 13321 Stephen Lewis Gp14 10 13559 John Bate Gp14 11 13948 David Edwards Gp14 12 13554 Club boat White Riot Gp14 14 Club boat Bob Gp14 15 Club boat Lottie Gp14 16 Club boat Insp Clouseau Gp14 17 Club boat 0 Gp14 18 Newc'e School CCF 3 Picos Racks 18A Newc'e School 3 Picos Racks 18B Richard Binns 3 boats 19 1627 Richard Binns Gp14 20 20 Club boat 0 Gp14 21 Red Richard Fish Wayfarer 22 Richard Binns Gp14 23 Steve Brooks Gp14 24 GEORGIA Bratt Gp14 25 12535 Suteera Fahey RS Vision 26 195 Ceiran Leigh GP14 27 Margaret Gosling GP14 28 8724 Brian Horbury Gp14 29 13066 Philip Whaite Enterprise 30 Not Known 0 GP14 30A 8376 Reserved for Pete Thomas Thomas 30B Sam Watson 0 30C Bev Morton ASC 30D Bev Morton ASC 30E Newc'e School CCF 0 Laser Pico 31 Newc'e School CCF 0 Laser Pico 31A Newc'e School CCF 0 Laser Pico 32 Jonathan Gorton Scorpion 33 Newc'e School CCF Argo 33A Newc'e School CCF Argo 33B Newc'e School CCF Argo 33C Access to Compound 0 0 33D Tim Elliot Hartley 12.2 34 Peter Owen GP14 35 11940 John Mcdonald Wayfarer 36 Nick Howell-Jones Wayfarer 37 9322 Page 1 of 5 Sean Barton Gp14 38 Martin Kirby Gp14 39 18673 Sam Barker Gp14 40 10459 Mark Price GP14 41 Gill Fox Gp14 42 6244 Andrew Dulla Gp14 43 Tracy Haden Gp14 44 11643 Rob Barlow GP14 -

Meet the Competitors: Annapolis YC Double-Handed Distance Race
Meet the Competitors: Annapolis YC Double-handed Distance Race R.J. Cooper & Courtney Cooper Cumberland are a brother and sister team from Oxford, Maryland and Panama City, Florida. They have sailed together throughout their youth as well as while on the Sailing Team for the University of Florida. The pair has teamed up for a bid to represent the United States and win gold at the 2024 Olympics in Paris. They will be sailing Tenacious owned by AYC member Carl Gitchell. Sail #501 Erik Haaland and Andrew Waters will be sailing the new Italia Yachts 9.98 sport boat named Vichingio (Viking). Erik Haaland is the Sales Director for Italia Yachts USA at David Walters Yachts. He has sailed his entire life and currently races on performance sport boats including the Farr 30, Melges 32 and J70. Andrew Waters is a Sail and Service Consultant at Quantum Sails in Annapolis. His professional sailing career began in South Africa and later the Caribbean and includes numerous wins in large regattas. Sail #17261 Ethan Johnson and Cat Chimney have sailing experience in dinghies, foiling skiffs, offshore racers and mini-Maxis. Ethan, a Southern Maryland native now living in NY is excited to be racing in home waters. Cat was born on Long Island, NY but spent time in Auckland, New Zealand. She has sailed with Olympians, America’s Cup sailors and Volvo Ocean Race sailors. Cat is Technical Specialist and Rigger at the prestigious Oakcliff Sailing where Ethan also works as the Training Program Director. Earlier this year Cat and Ethan teamed up to win the Oakcliff Double-handed Melges 24 Distance Race. -

Caretta Caretta) As Revealed by Stable Isotopes and Satellite Telemetry
Mar Biol (2012) 159:1255–1267 DOI 10.1007/s00227-012-1906-9 ORIGINAL PAPER Distribution of foraging habitats of male loggerhead turtles (Caretta caretta) as revealed by stable isotopes and satellite telemetry Mariela Pajuelo · Karen A. Bjorndal · Kimberly J. Reich · Michael D. Arendt · Alan B. Bolten Received: 13 October 2011 / Accepted: 20 February 2012 / Published online: 7 March 2012 © Springer-Verlag 2012 Abstract Most studies on the foraging ecology of logger- Introduction head turtles (Caretta caretta) have focused on adult females and juveniles. Little is known about the foraging Knowledge of foraging ground distribution of highly patterns of adult male loggerheads. We analyzed tissues for migratory animals is critical for understanding their forag- carbon and nitrogen stable isotopes (13C and 15N) from ing behavior and habitat use. IdentiWcation of key habitats 29 adult male loggerheads tracked with satellite transmit- helps not only to characterize life history features of popu- ters from one breeding area in Florida, USA, to evaluate lations (Block et al. 2001), but also to assess the impact of their foraging habitats in the Northwest Atlantic (NWA). threats that populations may face (Hays et al. 2003). Most Our study revealed large variations in 13C and 15N and a eVorts to identify key habitats and movement patterns have correlation between both 13C and 15N and the latitude to used Xipper tags (Limpus et al. 1992), genetic markers which the loggerheads traveled after the mating season, (Bolker et al. 2007), chemical analysis (Thorrold et al. thus reXecting a geographic pattern in the isotopic signa- 2001), and electronic tagging (Block et al. -

South Carolina Department of Natural Resources
FOREWORD Abundant fish and wildlife, unbroken coastal vistas, miles of scenic rivers, swamps and mountains open to exploration, and well-tended forests and fields…these resources enhance the quality of life that makes South Carolina a place people want to call home. We know our state’s natural resources are a primary reason that individuals and businesses choose to locate here. They are drawn to the high quality natural resources that South Carolinians love and appreciate. The quality of our state’s natural resources is no accident. It is the result of hard work and sound stewardship on the part of many citizens and agencies. The 20th century brought many changes to South Carolina; some of these changes had devastating results to the land. However, people rose to the challenge of restoring our resources. Over the past several decades, deer, wood duck and wild turkey populations have been restored, striped bass populations have recovered, the bald eagle has returned and more than half a million acres of wildlife habitat has been conserved. We in South Carolina are particularly proud of our accomplishments as we prepare to celebrate, in 2006, the 100th anniversary of game and fish law enforcement and management by the state of South Carolina. Since its inception, the South Carolina Department of Natural Resources (SCDNR) has undergone several reorganizations and name changes; however, more has changed in this state than the department’s name. According to the US Census Bureau, the South Carolina’s population has almost doubled since 1950 and the majority of our citizens now live in urban areas. -

December Channel 2002
OCTOBER 2003 TheCHANNEL Coconut Grove Sailing Club SKIPPER OF THE MONTH: 2003-2004 Officers C. J. Abell and Committee C J Abell started sailing at the CGSC when he was 9 Chairmen years old. It was a family thing. His dad, Charlie would Flag Officers take C J and his younger brother Michael to sail in an older Commodore ........................... Bud Price brothers boat, which had been sitting around the yard for Vice Commodore................ Jack Hamm quite some time. After a couple of weekends of sailing Rear Commodore...... Vladimir Stroleny they signed up for beginning lessons at the Miami Yacht Secretary ............................... Anne Platt Club. About a month later, the CGSC got its program Treasurer ..................... Jeffrey Zirulnick going again and C J began sailing in the CGSC racing and Fleet Officers summer programs. C J says that after the first week he Fleet Captain ........................Wil Bourne knew that he loved sailing. Fleet Chaplain .......... Brian C. Schofield Charlie got a small group to- Fleet Surgeon ....... Dr. Nicolaus Martens gether to begin a racing team. Board Members They would go out every weekend on both Saturday and Sunday Gonzalo Bellini Nick Martens with one of the parents on watch in a power boat. Jason Timmons, Jim Clark Felipe Mejia a past Opti Champion, became their coach. C J credits Jason for Steve Hawkins Jonathan Milley the inspiration to push himself to make and achieve goals and for Mike Lovelady Charles Rahn giving so much to the team, that he will always be remembered. Pat Gerry Marston Andrea Stringos, P.C. Downy, also became the CGSC racing coach and was instrumental in fine-tuning their skills. -

Reduced Mobility Is Associated with Compensatory Feeding and Increased Diet Breadth of Marine Crabs
MARINE ECOLOGY PROGRESS SERIES Published November 3 Mar Ecol Prog Ser Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs John J. Stachowicz*,Mark Hay8* University of North Carolina at Chapel Hill, Institute of Marine Sciences. Morehead City, North Carolina 28557. USA ABSTRACT: Direct effects of predation have been widely recognized as important in affecting prey population dynamics and evolution. However, less attention has been devoted to the consequences of indirect effects of predators on prey behavior. For example, to avoid predation many animals restrict their activities to physical refugia and adopt low-mobility Mestyles, yet the consequences of these anti- predator behaviors for foraging and diet selection are relatively unknown. In this study we examine the relationships between mobility, feeding preferences, and compensatory feeding for 3 species of marine decapod crabs feeding on seaweeds in North Carolina, USA. Low mobility and high site fidellty of crabs were associated with a broad, non-selective diet and compensatory feeding. The majid Mithrax forceps exhibited the lowest mobility, highest site fidelity, and least selective diet of the 3 species, whereas another majid Libinia dubia was intermehate in both rnobllity and selectivity, and the xanthid Panopeus herbstii had the greatest mobility and narrowest diet. Of these 3 crabs, only M. forceps com- pensated for low food quality by increasing consumption rates in single food-species feeding assays. This may be because M. forceps is resistant to (or tolerant of) seaweed chemical defenses, while other crab species are not. The ability to consume, and presumably subsist on, a wide variety of potential foods including those defended from more mobile consumers may facilitate a low-mobllity lifestyle, allowing the crab to minimize movement and reduce exposure to predators. -

Compiled Eel Abstracts: American Fisheries Society 2014 Annual Meeting August 18-21, 2014 Quebec City, Quebec, Canada [Compiled/Organized by R
Compiled Eel Abstracts: American Fisheries Society 2014 Annual Meeting August 18-21, 2014 Quebec City, Quebec, Canada [Compiled/organized by R. Wilson Laney, U.S. Fish and Wildlife Service, Raleigh, NC, USA] International Eel Symposium 2014: Are Eels Climbing Back up the Slippery Slope? Monday, August 18, 2014: 1:30 PM 206B (Centre des congrès de Québec // Québec City Convention Centre) Martin Castonguay, Institut Maurice-Lamontagne, Pêches et Océans Canada, Mont-Joli, QC, Canada David Cairns, Science Branch, Fisheries and Oceans Canada, Charlottetown, PE, Canada Guy Verreault, Ministere du Développement durable, de l'Environnement, de la Faune et des Parcs, Riviere-du-Loup, QC, Canada John Casselman, Dept. of Biology, Queen's University, Kingston, ON, Canada This talk will introduce the symposium. The first part will outline how the Symposium is structured, where to see Symposium posters, the panel, publication plans, etc. This would be followed by a brief outline of eel science and conservation issues, and how the Symposium will address these. I will then present with some level of detail the themes that the symposium will cover. The Introduction would also state the question that will be addressed by the panel discussion, and ask participants to keep this question in mind as they listen to the various talks. In closing, the introductory talk will outline the purpose of the Canada/USA Eel governance session that will be held at the very end of the meeting, after the Symposium panel. Publishing in the eel symposium proceedings - An orientation from the Editor-in- Chief of the ICES Journal of Marine Science Monday, August 18, 2014: 5:00 PM 206B (Centre des congrès de Québec // Québec City Convention Centre) Howard Browman, Marine Ecosystem Acoustics, Institute of Marine Research, Storebø, Norway I will provide symposium participants with a status report on the ICES Journal of Marine science. -

Smithsonian Contributions to Paleobiology • Number 90
SMITHSONIAN CONTRIBUTIONS TO PALEOBIOLOGY • NUMBER 90 Geology and Paleontology of the Lee Creek Mine, North Carolina, III Clayton E. Ray and David J. Bohaska EDITORS ISSUED MAY 112001 SMITHSONIAN INSTITUTION Smithsonian Institution Press Washington, D.C. 2001 ABSTRACT Ray, Clayton E., and David J. Bohaska, editors. Geology and Paleontology of the Lee Creek Mine, North Carolina, III. Smithsonian Contributions to Paleobiology, number 90, 365 pages, 127 figures, 45 plates, 32 tables, 2001.—This volume on the geology and paleontology of the Lee Creek Mine is the third of four to be dedicated to the late Remington Kellogg. It includes a prodromus and six papers on nonmammalian vertebrate paleontology. The prodromus con tinues the historical theme of the introductions to volumes I and II, reviewing and resuscitat ing additional early reports of Atlantic Coastal Plain fossils. Harry L. Fierstine identifies five species of the billfish family Istiophoridae from some 500 bones collected in the Yorktown Formation. These include the only record of Makairapurdyi Fierstine, the first fossil record of the genus Tetrapturus, specifically T. albidus Poey, the second fossil record of Istiophorus platypterus (Shaw and Nodder) and Makaira indica (Cuvier), and the first fossil record of/. platypterus, M. indica, M. nigricans Lacepede, and T. albidus from fossil deposits bordering the Atlantic Ocean. Robert W. Purdy and five coauthors identify 104 taxa from 52 families of cartilaginous and bony fishes from the Pungo River and Yorktown formations. The 10 teleosts and 44 selachians from the Pungo River Formation indicate correlation with the Burdigalian and Langhian stages. The 37 cartilaginous and 40 bony fishes, mostly from the Sunken Meadow member of the Yorktown Formation, are compatible with assignment to the early Pliocene planktonic foraminiferal zones N18 or N19. -

Indicators of Climate Change and Social Vulnerability in Fishing Dependent Communities Along the Eastern and Gulf Coasts of the U.S
University of New Haven Digital Commons @ New Haven Biology and Environmental Science Faculty Biology and Environmental Science Publications 2016 Indicators of Climate Change and Social Vulnerability in Fishing Dependent Communities Along the Eastern and Gulf Coasts of the U.S. Marine Policy Lisa Colburn NOAA Michael Jepson NOAA Changhua Weng NOAA Tarsila Seara University of New Haven, [email protected] Jeremy Weiss NOAA Follow this and additional works at: http://digitalcommons.newhaven.edu/biology-facpubs Part of the Biology Commons, and the Ecology and Evolutionary Biology Commons Publisher Citation Colburn, L. L., M. Jepson, C. Weng, T. Seara, J. Weiss, and J. Hare (2016). Indicators of climate change and social vulnerability in fishing dependent communities along the Eastern and Gulf Coasts of the U.S. Marine Policy. Published online 13 May 2016 Comments This is the in-press corrected proof posted by Marine Policy in advance of the final publication. This article is published open-access. Marine Policy ∎ (∎∎∎∎) ∎∎∎–∎∎∎ Contents lists available at ScienceDirect Marine Policy journal homepage: www.elsevier.com/locate/marpol Indicators of climate change and social vulnerability in fishing dependent communities along the Eastern and Gulf Coasts of the United States Lisa L. Colburn a,n, Michael Jepson b, Changhua Weng a, Tarsila Seara c,d, Jeremy Weiss e, Jonathan A. Hare f a NOAA Fisheries, Northeast Fisheries Science Center, Social Sciences Branch, 28 Tarzwell Dr., Narragansett, RI 02882, USA b NOAA Fisheries, Southeast Regional Office, -

Marine Ecology Progress Series 513:143
The following supplement accompanies the article Commercial trawling in seagrass beds: bycatch and long-term trends in effort of a major shrimp fishery C. D. Stallings1,2,*, J. P. Brower1,3, J. M. Heinlein Loch1, A. Mickle1 1Florida State University Coastal and Marine Laboratory, 3618 Coastal Highway 98, St. Teresa, Florida 32358-2702, USA 2College of Marine Science, University of South Florida, 140 Seventh Avenue South, St. Petersburg, Florida 33701-5016, USA 3Department of Biology, San Diego State University, 5500 Campanile Dive, San Diego, California 92182-4614, USA *Corresponding author: [email protected] Marine Ecology Progress Series 513: 143–153 (2014) Supplement. Catch comparison between rollerframe and otter trawls (Table S1), and photographs of potential predators on post-release by-catch from a commercial bait-shrimp trawler (Fig. S1) Table S1. Catch composition from fishery-independent sampling with paired rollerframe (n = 52 tows) and otter trawls (n = 51 tows). Taxa are listed from the most to least abundant captured by rollerframe trawls. The mean (± SE) densities (number catch per 100 m2) of captured animals are provided for Size Classes 1 to 6 Species Common Name Gear No. L1 L 2 L 3 L4 L 5 L 6 caught (1–25 mm) (26–50 mm) (51–75 mm) (76–100 mm) (101–150 mm) (>150 mm) Arthropoda Tozeuma Arrow Shrimp rollerframe 90,146 327.30 (73.22) 0.03 (0.03) – – – – carolinense otter 38,510 137.29 (44.29) – – – – – Farfantepenaeus Pink Shrimp rollerframe 27,124 96.10 (10.71) 2.38 (0.42) 0.02 (0.02) – – – duorarum otter 3,474 11.76 (2.16)