Life Style, Eye Design and the Optimum Strategy of Spatial and Temporal Summation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Animal Eyes and the Darwinian Theory of the Evolution of the Human
Animal Eyes We can learn a lot from the wonder of, and the wonder in, animal eyes. Aldo Leopold a pioneer in the conservation movement did. He wrote in Thinking like a Mountain, “We reached the old wolf in time to watch a fierce green fire dying in her eyes. I realized then, and have known ever since, that there was something new to me in those eyes – something known only to her and to the mountain. I was young then, and full of trigger-itch; I thought that because fewer wolves meant more deer, that no wolves would mean hunters’ paradise. But after seeing the green fire die, I sensed that neither the wolf nor the mountain agreed with such a view.” For Aldo Leopold, the green fire in the wolf’s eyes symbolized a new way of seeing our place in the world, and with his new insight, he provided a new ethical perspective for the environmental movement. http://vimeo.com/8669977 Light contains information about the environment, and animals without eyes can make use of the information provided by environmental light without forming an image. Euglena, a single-celled organism that did not fit nicely into Carl Linnaeus’ two kingdom system of classification, quite clearly responds to light. Its plant-like nature responds to light by photosynthesizing and its animal- like nature responds to light by moving to and staying in the light. Light causes an increase in the swimming speed, a response known as 165 photokinesis. Light also causes another response in Euglena, known as an accumulation response (phototaxis). -

Spatial Restriction of Light Adaptation Inactivation in Fly Photoreceptors and Mutation-Induced
The Journal of Neuroscience, April 1991, 7 f(4): 900-909 Spatial Restriction of Light Adaptation and Mutation-Induced Inactivation in Fly Photoreceptors Baruch Minke and Richard Payne” Department of Neuroscience, The Howard Hughes Medical Institute, The Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 The spatial spread within fly photoreceptors of 2 forms of We describe simple preparations of the retinas of the housefly, desensitization by bright light have been investigated: the Musca domestica, and the sheepblowfly, Lucilia cuprina, that natural process of light adaptation in normal Musca photo- allow recording from individual ommatidia in vitro, using a receptors and a receptor-potential inactivation in the no- suction pipette similar to that used to record from rod photo- steady-state (nss) mutant of the sheep blowfly Lucilia. The receptors of the vertebrate retina (Baylor et al., 1979; for a suction-electrode method used for recording from verte- preliminary use of this method, see also Becker et al., 1987). brate rods was applied to fly ommatidia. A single ommatid- The suction-electrode method has 2 advantages when applied ium in vitro was partially sucked into a recording pipette. to fly photoreceptors. First, the method might prove to be more Illumination of the portion of the ommatidium within the pi- convenient than the use of intracellular microelectrodes when pette resulted in a flow of current having a wave form similar recording from small photoreceptors, such as those of Drosoph- to that of the receptor potential and polarity consistent with ila. Recordings from Drosophila are needed to perform func- current flow into the illuminated region of the photorecep- tional tests on mutant flies. -

Spectral Sensitivities of Wolf Spider Eyes
=ORNELL UNIVERSITY ~',lC.V~;AL ~ULLE(.~E DEF'ARY~,:ENT OF P~-I:~IOLOGY 1300 YORK AVE.~UE NEW YORK, N.Y. Spectral Sensitivities of Wolf Spider Eyes ROBERT D. DBVOE, RALPH J. W. SMALL, and JANIS E. ZVARGULIS From the Department of Physiology, The Johns Hopkins University School of Medicine, Baltimore, Maryland 21205 ABSTRACT ERG's to spectral lights were recorded from all eyes of intact wolf spiders. Secondary eyes have maximum relative sensitivities at 505-510 nm which are unchanged by chromatic adaptations. Principal eyes have ultraviolet sensitivities which are 10 to 100 times greater at 380 nm than at 505 nm. How- ever, two animals' eyes initially had greater blue-green sensitivities, then in 7 to 10 wk dropped 4 to 6 log units in absolute sensitivity in the visible, less in the ultraviolet. Chromatic adaptations of both types of principal eyes hardly changed relative spectral sensitivities. Small decreases in relative sensitivity in the visible with orange adaptations were possibly retinomotor in origin. Second peaks in ERG waveforms were elicited from ultraviolet-adapted principal eyes by wavelengths 400 nm and longer, and from blue-, yellow-, and orange- adapted secondary eyes by wavelengths 580 nm and longer. The second peaks in waveforms were most likely responses of unilluminated eyes to scattered light. It is concluded that both principal and secondary eyes contain cells with a visual pigment absorbing maximally at 505-510 nm. The variable absolute and ultraviolet sensitivities of principal eyes may be due to a second pigment in the same cells or to an ultraviolet-absorbing accessory pigment which excites the 505 nm absorbing visual pigment by radiationless energy transfer. -

Lafranca Moth Article.Pdf
What you may not know about... MScientific classificationoths Kingdom: Animalia Phylum: Arthropoda Class: Insecta Photography and article written by Milena LaFranca order: Lepidoptera [email protected] At roughly 160,000, there are nearly day or nighttime. Butterflies are only above: scales on moth wing, shot at 2x above: SEM image of individual wing scale, 1500x ten times the number of species of known to be diurnal insects and moths of moths have thin butterfly-like of microscopic ridges and bumps moths compared to butterflies, which are mostly nocturnal insects. So if the antennae but they lack the club ends. that reflect light in various angles are in the same order. While most sun is out, it is most likely a butterfly and Moths utilize a wing-coupling that create iridescent coloring. moth species are nocturnal, there are if the moon is out, it is definitely a moth. mechanism that includes two I t i s c o m m o n f o r m o t h w i n g s t o h a v e some that are crepuscular and others A subtler clue in butterfly/moth structures, the retinaculum and patterns that are not in the human that are diurnal. Crepuscular meaning detection is to compare the placement the frenulum. The frenulum is a visible light spectrum. Moths have that they are active during twilight of their wings at rest. Unless warming spine at the base of the hind wing. the ability to see in ultra-violet wave hours. Diurnal themselves, The retinaculum is a loop on the lengths. -

Auditory Experience Controls the Maturation of Song Discrimination and Sexual Response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi*
RESEARCH ARTICLE Auditory experience controls the maturation of song discrimination and sexual response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi* Graduate School of Science, Nagoya University, Nagoya, Japan Abstract In birds and higher mammals, auditory experience during development is critical to discriminate sound patterns in adulthood. However, the neural and molecular nature of this acquired ability remains elusive. In fruit flies, acoustic perception has been thought to be innate. Here we report, surprisingly, that auditory experience of a species-specific courtship song in developing Drosophila shapes adult song perception and resultant sexual behavior. Preferences in the song-response behaviors of both males and females were tuned by social acoustic exposure during development. We examined the molecular and cellular determinants of this social acoustic learning and found that GABA signaling acting on the GABAA receptor Rdl in the pC1 neurons, the integration node for courtship stimuli, regulated auditory tuning and sexual behavior. These findings demonstrate that maturation of auditory perception in flies is unexpectedly plastic and is acquired socially, providing a model to investigate how song learning regulates mating preference in insects. DOI: https://doi.org/10.7554/eLife.34348.001 Introduction Vocal learning in infants or juvenile birds relies heavily on the early experience of the adult conspe- cific sounds. In humans, early language input is necessary to form the ability of phonetic distinction *For correspondence: and pattern detection in the phase of auditory learning (Doupe and Kuhl, 1999; Kuhl, 2004). [email protected] Because of the strong parallels between speech acquisition of humans and song learning of song- Competing interests: The birds, and the difficulties to investigate the neural mechanisms of human early auditory memory at authors declare that no cellular resolution, songbirds have been used as a predominant model in studying memory formation competing interests exist. -

Seeing Through Moving Eyes
bioRxiv preprint doi: https://doi.org/10.1101/083691; this version posted June 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Seeing through moving eyes - microsaccadic information sampling provides 2 Drosophila hyperacute vision 3 4 Mikko Juusola1,2*‡, An Dau2‡, Zhuoyi Song2‡, Narendra Solanki2, Diana Rien1,2, David Jaciuch2, 5 Sidhartha Dongre2, Florence Blanchard2, Gonzalo G. de Polavieja3, Roger C. Hardie4 and Jouni 6 Takalo2 7 8 1National Key laboratory of Cognitive Neuroscience and Learning, Beijing, Beijing Normal 9 University, Beijing 100875, China 10 2Department of Biomedical Science, University of Sheffield, Sheffield S10 T2N, UK 11 3Champalimaud Neuroscience Programme, Champalimaud Center for the Unknown, Lisbon, 12 Portugal 13 4Department of Physiology Development and Neuroscience, Cambridge University, Cambridge CB2 14 3EG, UK 15 16 *Correspondence to: [email protected] 17 ‡ Equal contribution 18 19 Small fly eyes should not see fine image details. Because flies exhibit saccadic visual behaviors 20 and their compound eyes have relatively few ommatidia (sampling points), their photoreceptors 21 would be expected to generate blurry and coarse retinal images of the world. Here we 22 demonstrate that Drosophila see the world far better than predicted from the classic theories. 23 By using electrophysiological, optical and behavioral assays, we found that R1-R6 24 photoreceptors’ encoding capacity in time is maximized to fast high-contrast bursts, which 25 resemble their light input during saccadic behaviors. Whilst over space, R1-R6s resolve moving 26 objects at saccadic speeds beyond the predicted motion-blur-limit. -
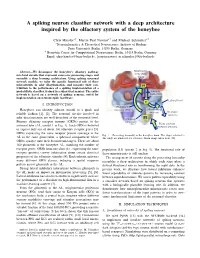
A Spiking Neuron Classifier Network with a Deep Architecture Inspired By
A spiking neuron classifier network with a deep architecture inspired by the olfactory system of the honeybee Chris Hausler¨ ∗†, Martin Paul Nawrot∗† and Michael Schmuker∗† ∗Neuroinformatics & Theoretical Neuroscience, Institute of Biology Freie Universitat¨ Berlin, 14195 Berlin, Germany † Bernstein Center for Computational Neuroscience Berlin, 10115 Berlin, Germany Email: [email protected], fmartin.nawrot, [email protected] Abstract—We decompose the honeybee’s olfactory pathway Mushroom into local circuits that represent successive processing stages and body (MB) calyx resemble a deep learning architecture. Using spiking neuronal network models, we infer the specific functional role of these microcircuits in odor discrimination, and measure their con- 2 tribution to the performance of a spiking implementation of a probabilistic classifier, trained in a supervised manner. The entire Kenyon network is based on a network of spiking neurons, suited for Cells (KC) implementation on neuromorphic hardware. Lateral horn 3 I. INTRODUCTION Projection Honeybees can identify odorant stimuli in a quick and neurons (PN) MB-extrinsic neurons To motor reliable fashion [1], [2]. The neuronal circuits involved in neurons odor discrimination are well described at the structural level. 1 Antennal Primary olfactory receptor neurons (ORNs) project to the lobe (AL) From odorant antennal lobe (AL, circuit 1 in Fig. 1). Each ORN is believed receptor neurons to express only one of about 160 olfactory receptor genes [3]. ORNs expressing the same receptor protein converge in the Fig. 1. Processing hierarchy in the honeybee brain. The stages relevant to AL in the same glomerulus, a spherical compartment where this study are numbered for reference. -

Introduction; Environment & Review of Eyes in Different Species
The Biological Vision System: Introduction; Environment & Review of Eyes in Different Species James T. Fulton https://neuronresearch.net/vision/ Abstract: Keywords: Biological, Human, Vision, phylogeny, vitamin A, Electrolytic Theory of the Neuron, liquid crystal, Activa, anatomy, histology, cytology PROCESSES IN BIOLOGICAL VISION: including, ELECTROCHEMISTRY OF THE NEURON Introduction 1- 1 1 Introduction, Phylogeny & Generic Forms 1 “Vision is the process of discovering from images what is present in the world, and where it is” (Marr, 1985) ***When encountering a citation to a Section number in the following material, the first numeric is a chapter number. All cited chapters can be found at https://neuronresearch.net/vision/document.htm *** 1.1 Introduction While the material in this work is designed for the graduate student undertaking independent study of the vision sensory modality of the biological system, with a certain amount of mathematical sophistication on the part of the reader, the major emphasis is on specific models down to specific circuits used within the neuron. The Chapters are written to stand-alone as much as possible following the block diagram in Section 1.5. However, this requires frequent cross-references to other Chapters as the analyses proceed. The results can be followed by anyone with a college degree in Science. However, to replicate the (photon) Excitation/De-excitation Equation, a background in differential equations and integration-by-parts is required. Some background in semiconductor physics is necessary to understand how the active element within a neuron operates and the unique character of liquid-crystalline water (the backbone of the neural system). The level of sophistication in the animal vision system is quite remarkable. -

1P MON. PM Cortex and Subcortical Auditory Nuclei
dynamic role for the vestibular system in orientation and flight control. Laboratory studies of flight behavior under illuminated and dark conditions in both static and rotating obstacle tests were carried out while administering heavy water ͑D2O͒ to bats to impair their vestibular inputs. Eptesicus carried out complex maneuvers through both fixed arrays of wires and a rotating obstacle array using both vision and echolocation, or when guided by echolocation alone. When treated with D2O in combination with lack of visual cues, bats showed considerable decrements in performance. These data indicate that big brown bats use both vision and echolocation to provide spatial registration for head position information generated by the vestibular system. 2:55 1pAB3. Bat’s auditory system: Corticofugal feedback and plasticity. Nobuo Suga ͑Dept. of Biol., Washington Univ., One Brookings Dr., St. Louis, MO 63130͒ The auditory system of the mustached bat consists of physiologically distinct subdivisions for processing different types of biosonar information. It was found that the corticofugal ͑descending͒ auditory system plays an important role in improving and adjusting auditory signal processing. Repetitive acoustic stimulation, cortical electrical stimulation or auditory fear conditioning evokes plastic changes of the central auditory system. The changes are based upon egocentric selection evoked by focused positive feedback associated with lateral inhibition. Focal electric stimulation of the auditory cortex evokes short-term changes in the auditory 1p MON. PM cortex and subcortical auditory nuclei. An increase in a cortical acetylcholine level during the electric stimulation changes the cortical changes from short-term to long-term. There are two types of plastic changes ͑reorganizations͒: centripetal best frequency shifts for expanded reorganization of a neural frequency map and centrifugal best frequency shifts for compressed reorganization of the map. -

The Biochromes ) 1.2
FORSCHUNG 45 CHIMIA 49 (1995) Nr. 3 (Miirz) Chim;a 49 (1995) 45-68 quire specific molecules, pigments or dyes © Neue Schweizerische Chemische Gesellschaft (biochromes) or systems containing them, /SSN 0009-4293 to absorb the light energy. Photoprocesses and colors are essential for life on earth, and without these biochromes and the photophysical and photochemical interac- tions, life as we know it would not have The Function of Natural been possible [1][2]. a Colorants: The Biochromes ) 1.2. Notation The terms colorants, dyes, and pig- ments ought to be used in the following way [3]: Colorants are either dyes or pig- Hans-Dieter Martin* ments, the latter being practically insolu- ble in the media in which they are applied. Indiscriminate use of these terms is fre- Abstract. The colors of nature belong undoubtedly to the beautiful part of our quently to be found in literature, but in environment. Colors always fascinated humans and left them wonderstruck. But the many biological systems it is not possible trivial question as to the practical application of natural colorants led soon and at all to make this differentiation. The consequently to coloring and dyeing of objects and humans. Aesthetical, ritual and coloring compounds of organisms have similar aspects prevailed. This function of dyes and pigments is widespread in natl)re. been referred to as biochromes, and this The importance of such visual-effective dyes is obvious: they support communication seems to be a suitable expression for a between organisms with the aid of conspicuous optical signals and they conceal biological colorant, since it circumvents revealing ones, wl,1eninconspicuosness can mean survival. -

Portia Perceptions: the Umwelt of an Araneophagic Jumping Spider
Portia Perceptions: The Umwelt of an Araneophagic Jumping 1 Spider Duane P. Harland and Robert R. Jackson The Personality of Portia Spiders are traditionally portrayed as simple, instinct-driven animals (Savory, 1928; Drees, 1952; Bristowe, 1958). Small brain size is perhaps the most compelling reason for expecting so little flexibility from our eight-legged neighbors. Fitting comfortably on the head of a pin, a spider brain seems to vanish into insignificance. Common sense tells us that compared with large-brained mammals, spiders have so little to work with that they must be restricted to a circumscribed set of rigid behaviors, flexibility being a luxury afforded only to those with much larger central nervous systems. In this chapter we review recent findings on an unusual group of spiders that seem to be arachnid enigmas. In a number of ways the behavior of the araneophagic jumping spiders is more comparable to that of birds and mammals than conventional wisdom would lead us to expect of an arthropod. The term araneophagic refers to these spiders’ preference for other spiders as prey, and jumping spider is the common English name for members of the family Saltici- dae. Although both their common and the scientific Latin names acknowledge their jumping behavior, it is really their unique, complex eyes that set this family of spiders apart from all others. Among spiders (many of which have very poor vision), salticids have eyes that are by far the most specialized for resolving fine spatial detail. We focus here on the most extensively studied genus, Portia. Before we discuss the interrelationship between the salticids’ uniquely acute vision, their predatory strategies, and their apparent cognitive abilities, we need to offer some sense of what kind of animal a jumping spider is; to do this, we attempt to offer some insight into what we might call Portia’s personality. -

Vision-In-Arthropoda.Pdf
Introduction Arthropods possess various kinds of sensory structures which are sensitive to different kinds of stimuli. Arthropods possess simple as well as compound eyes; the latter evolved in Arthropods and are found in no other group of animals. Insects that possess both types of eyes: simple and compound. Photoreceptors: sensitive to light Photoreceptor in Arthropoda 1. Simple Eyes 2. Compound Eyes 1. Simple Eyes in Arthropods - Ocelli The word ocelli are derived from the Latin word ocellus which means little eye. Ocelli are simple eyes which comprise of single lens for collecting and focusing light. Arthropods possess two kinds of ocelli a) Dorsal Ocelli b) Lateral Ocelli (Stemmata) Dorsal Ocellus - Dorsal ocelli are found on the dorsal or front surface of the head of nymphs and adults of several hemimetabolous insects. These are bounded by compound eyes on lateral sides. Dorsal ocelli are not present in those arthropods which lack compound eyes. • Dorsal ocellus has single corneal lens which covers a number of sensory rod- like structures, rhabdome. • The ocellar lens may be curved, for example in bees, locusts and dragonflies; or flat as in cockroaches. • It is sensitive to a wide range of wavelengths and shows quick response to changes in light intensity. • It cannot form an image and is unable to recognize the object. Lateral Ocellus - Stemmata Lateral ocelli, It is also known as stemmata. They are the only eyes in the larvae of holometabolous and certain adult insects such as spring tails, silver fish, fleas and stylops. These are called lateral eyes because they are always present in the lateral region of the head.