A Multiscale View of the Phanerozoic Fossil Record Reveals the Three Major Biotic Transitions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Sponge Evolution: a Review and Phylogenetic Framework
Available online at www.sciencedirect.com ScienceDirect Palaeoworld 27 (2018) 1–29 Review Early sponge evolution: A review and phylogenetic framework a,b,∗ a Joseph P. Botting , Lucy A. Muir a Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, 39 East Beijing Road, Nanjing 210008, China b Department of Natural Sciences, Amgueddfa Cymru — National Museum Wales, Cathays Park, Cardiff CF10 3LP, UK Received 27 January 2017; received in revised form 12 May 2017; accepted 5 July 2017 Available online 13 July 2017 Abstract Sponges are one of the critical groups in understanding the early evolution of animals. Traditional views of these relationships are currently being challenged by molecular data, but the debate has so far made little use of recent palaeontological advances that provide an independent perspective on deep sponge evolution. This review summarises the available information, particularly where the fossil record reveals extinct character combinations that directly impinge on our understanding of high-level relationships and evolutionary origins. An evolutionary outline is proposed that includes the major early fossil groups, combining the fossil record with molecular phylogenetics. The key points are as follows. (1) Crown-group sponge classes are difficult to recognise in the fossil record, with the exception of demosponges, the origins of which are now becoming clear. (2) Hexactine spicules were present in the stem lineages of Hexactinellida, Demospongiae, Silicea and probably also Calcarea and Porifera; this spicule type is not diagnostic of hexactinellids in the fossil record. (3) Reticulosans form the stem lineage of Silicea, and probably also Porifera. (4) At least some early-branching groups possessed biminerallic spicules of silica (with axial filament) combined with an outer layer of calcite secreted within an organic sheath. -

Evolutionary Patterns of Trilobites Across the End Ordovician Mass Extinction
Evolutionary Patterns of Trilobites Across the End Ordovician Mass Extinction by Curtis R. Congreve B.S., University of Rochester, 2006 M.S., University of Kansas, 2008 Submitted to the Department of Geology and the Faculty of the Graduate School of The University of Kansas in partial fulfillment on the requirements for the degree of Doctor of Philosophy 2012 Advisory Committee: ______________________________ Bruce Lieberman, Chair ______________________________ Paul Selden ______________________________ David Fowle ______________________________ Ed Wiley ______________________________ Xingong Li Defense Date: December 12, 2012 ii The Dissertation Committee for Curtis R. Congreve certifies that this is the approved Version of the following thesis: Evolutionary Patterns of Trilobites Across the End Ordovician Mass Extinction Advisory Committee: ______________________________ Bruce Lieberman, Chair ______________________________ Paul Selden ______________________________ David Fowle ______________________________ Ed Wiley ______________________________ Xingong Li Accepted: April 18, 2013 iii Abstract: The end Ordovician mass extinction is the second largest extinction event in the history or life and it is classically interpreted as being caused by a sudden and unstable icehouse during otherwise greenhouse conditions. The extinction occurred in two pulses, with a brief rise of a recovery fauna (Hirnantia fauna) between pulses. The extinction patterns of trilobites are studied in this thesis in order to better understand selectivity of the -

Early Paleozoic Life & Extinctions (Part 1)
NJU Course Extinctions: Past, Present & Future Prof. Norman MacLeod School of Earth Sciences & Engineering, Nanjing University Extinctions: Past, Present & Future Extinctions: Past, Present & Future Course Syllabus (Revised) Section Week Title Introduction 1 Course Introduction, Intro. To Extinction Introduction 2 History of Extinction Studies Introduction 3 Evolution, Fossils, Time & Extinction Precambrian Extinctions 4 Origin of Life & Precambrian Extionctions Paleozoic Extinctions 5 Early Paleozoic World & Extinctions Paleozoic Extinctions 6 Middle Paleozoic World & Extinctions Paleozoic Extinctions 7 Late Paleozoic World & Extinctions Assessment 8 Mid-Term Examination Mesozoic Extinctions 9 Triassic-Jurassic World & Extinctions Mesozoic Extinctions 10 Labor Day Holiday Cenozoic Extinctions 11 Cretaceous World & Extinctions Cenozoic Extinctions 12 Paleogene World & Extinctions Cenozoic Extinctions 13 Neogene World & Extinctions Modern Extinctions 14 Quaternary World & Extinctions Modern Extinctions 15 Modern World: Floras, Faunas & Environment Modern Extinctions 16 Modern World: Habitats & Organisms Assessment 17 Final Examination Early Paleozoic World, Life & Extinctions Norman MacLeod School of Earth Sciences & Engineering, Nanjing University Early Paleozoic World, Life & Extinctions Objectives Understand the structure of the early Paleozoic world in terms of timescales, geography, environ- ments, and organisms. Understand the structure of early Paleozoic extinction events. Understand the major Paleozoic extinction drivers. Understand -

Diversity Partitioning During the Cambrian Radiation
Diversity partitioning during the Cambrian radiation Lin Naa,1 and Wolfgang Kiesslinga,b aGeoZentrum Nordbayern, Paleobiology and Paleoenvironments, Friedrich-Alexander-Universität Erlangen-Nürnberg, 91054 Erlangen, Germany; and bMuseum für Naturkunde, Leibniz Institute for Research on Evolution and Biodiversity at the Humboldt University Berlin, 10115 Berlin, Germany Edited by Douglas H. Erwin, Smithsonian National Museum of Natural History, Washington, DC, and accepted by the Editorial Board March 10, 2015 (received for review January 2, 2015) The fossil record offers unique insights into the environmental and Results geographic partitioning of biodiversity during global diversifica- Raw gamma diversity exhibits a strong increase in the first three tions. We explored biodiversity patterns during the Cambrian Cambrian stages (informally referred to as early Cambrian in this radiation, the most dramatic radiation in Earth history. We as- work) (Fig. 1A). Gamma diversity dropped in Stage 4 and de- sessed how the overall increase in global diversity was partitioned clined further through the rest of the Cambrian. The pattern is between within-community (alpha) and between-community (beta) robust to sampling standardization (Fig. 1B) and insensitive to components and how beta diversity was partitioned among environ- including or excluding the archaeocyath sponges, which are po- ments and geographic regions. Changes in gamma diversity in the tentially oversplit (16). Alpha and beta diversity increased from Cambrian were chiefly driven by changes in beta diversity. The the Fortunian to Stage 3, and fluctuated erratically through the combined trajectories of alpha and beta diversity during the initial following stages (Fig. 2). Our estimate of alpha (and indirectly diversification suggest low competition and high predation within beta) diversity is based on the number of genera in published communities. -

Associated Organisms Inhabiting the Calcareous Sponge Clathrina Lutea in La Parguera Natural
bioRxiv preprint doi: https://doi.org/10.1101/596429; this version posted April 3, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Associated organisms inhabiting the calcareous sponge Clathrina lutea in La Parguera Natural 2 Reserve, Puerto Rico 3 4 Jaaziel E. García-Hernández1,2*, Nicholas M. Hammerman2,3*, Juan J. Cruz-Motta2 & Nikolaos V. 5 Schizas2 6 * These authors contributed equally 7 1University of Puerto Rico at Mayagüez, Department of Biology, PO Box 9000, Mayagüez, PR 8 00681 9 2University of Puerto Rico at Mayagüez, Department of Marine Sciences, Marine Genomic 10 Biodiversity Laboratory, PO Box 9000, Mayagüez, PR 00681 11 3School of Biological Sciences, University of Queensland, Gehrmann Laboratories, Level 8, 12 Research Road, St Lucia, QLD 4072, Australia 13 14 15 Nikolaos V. Schizas, [email protected], FAX: 787-899-5500 16 17 Running Head: Infauna of the calcareous sponge Clathrina lutea 18 19 20 1 bioRxiv preprint doi: https://doi.org/10.1101/596429; this version posted April 3, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 21 ABSTRACT 22 Sponges provide an array of ecological services and benefits for Caribbean coral reefs. They 23 function as habitats for a bewildering variety of species, however limited attention has been paid 24 in the systematics and distribution of sponge-associated fauna in the class Calcarea or for that 25 matter of sponges in the Caribbean. -

The Paleoecology and Biogeography of Ordovician Edrioasteroids
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2011 The Paleoecology and Biogeography of Ordovician Edrioasteroids Rene Anne Lewis University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Paleontology Commons Recommended Citation Lewis, Rene Anne, "The Paleoecology and Biogeography of Ordovician Edrioasteroids. " PhD diss., University of Tennessee, 2011. https://trace.tennessee.edu/utk_graddiss/1094 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Rene Anne Lewis entitled "The Paleoecology and Biogeography of Ordovician Edrioasteroids." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Geology. Michael L. McKinney, Major Professor We have read this dissertation and recommend its acceptance: Colin D. Sumrall, Linda C. Kah, Arthur C. Echternacht Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) THE PALEOECOLOGY AND BIOGEOGRAPHY OF ORDOVICIAN EDRIOASTEROIDS A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville René Anne Lewis August 2011 Copyright © 2011 by René Anne Lewis All rights reserved. -
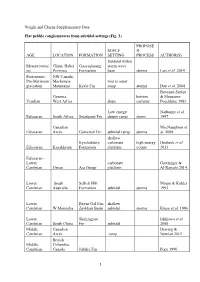
Wright and Cherns Supplementary Data Flat
Wright and Cherns Supplementary Data Flat pebble conglomerates from subtidal settings (Fig. 2) PROPOSE SHELF D AGE LOCATION FORMATION SETTING PROCESS AUTHOR(S) Subtidal within Mesoproteroz China, Hebei Gaoyuzhuang storm wave oic Province Formation base storms Luo et al. 2014 Proterozoic, NW Canada, Pre-Marinoan Mackenzie mid to outer glaciation Mountains Keele Fm ramp storms Day et al. 2004 Bertrand-Sarfati Gourma, bottom & Moussine- Vendian West Africa slope currents' Pouchkine 1983 Low energy Narbonne et al. Ediacaran South Africa Swartpunt Fm deeper ramp storm 1997 Canadian MacNaughton et Ediacaran Arctic Gametrail Fm subtidal ramp storms al. 2008 shallow Kyrshabakty carbonate high energy Heubeck et al. Ediacaran Kazakhstan Formation platform events 2013 Ediacaran - Lower carbonate Grotzinger & Cambrian Oman Ara Group platform Al-Rawahi 2014 Lower South Sellick Hill Mount & Kidder Cambrian Australia Formation subtidal storms 1993 Lower Bayan Gol Fm, shallow Cambrian W Mongolia Zavkhan Basin subtidal storms Kruse et al. 1996 Lower Shuijingtuo Ishikawa et al. Cambrian South China Fm subtidal 2008 Middle Canadian Dewing & Cambrian Arctic ramp Nowlan 2012 British Middle Columbia, Cambrian Canada Jubilee Fm Pope 1990 1 Middle Pratt & Cambrian Argentina La Laja Fm subtidal shelf tsunamis Bordonaro 2007 low energy Middle shallow Cambrian Australia Ranken Lst subtidal storms Kruse 1996 Middle Wyoming, Upper Gros Cambrian USA Ventre Shale Csonka 2009 middle upper Middle W Utah, upper Wheeler, carbonate belt - Cambrian USA Marjum fms subtidal shelf Robison 1964 Middle- Upper Supratidal to Cambrian NW China subtidal fpc storms Liang et al. 1993 Ust’- Brus,Labaz, Middle Orakta, Cambrian - Kulyumbe, Lower Ujgur and Iltyk Kouchinsky et Ordovician Siberia fms al. -

End-Permian Mass Extinction in the Oceans: an Ancient Analog for the Twenty-First Century?
EA40CH05-Payne ARI 23 March 2012 10:24 End-Permian Mass Extinction in the Oceans: An Ancient Analog for the Twenty-First Century? Jonathan L. Payne1 and Matthew E. Clapham2 1Department of Geological and Environmental Sciences, Stanford University, Stanford, California 94305; email: [email protected] 2Department of Earth and Planetary Sciences, University of California, Santa Cruz, California 95064; email: [email protected] Annu. Rev. Earth Planet. Sci. 2012. 40:89–111 Keywords First published online as a Review in Advance on ocean acidification, evolution, isotope geochemistry, volcanism, January 3, 2012 biodiversity The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The greatest loss of biodiversity in the history of animal life occurred at the 10.1146/annurev-earth-042711-105329 end of the Permian Period (∼252 million years ago). This biotic catastro- Annu. Rev. Earth Planet. Sci. 2012.40:89-111. Downloaded from www.annualreviews.org Copyright c 2012 by Annual Reviews. phe coincided with an interval of widespread ocean anoxia and the eruption All rights reserved of one of Earth’s largest continental flood basalt provinces, the Siberian by Stanford University - Main Campus Robert Crown Law Library on 06/04/12. For personal use only. 0084-6597/12/0530-0089$20.00 Traps. Volatile release from basaltic magma and sedimentary strata dur- ing emplacement of the Siberian Traps can account for most end-Permian paleontological and geochemical observations. Climate change and, per- haps, destruction of the ozone layer can explain extinctions on land, whereas changes in ocean oxygen levels, CO2, pH, and temperature can account for extinction selectivity across marine animals. -

Asteroid Impact, Not Volcanism, Caused the End-Cretaceous Dinosaur Extinction
Asteroid impact, not volcanism, caused the end-Cretaceous dinosaur extinction Alfio Alessandro Chiarenzaa,b,1,2, Alexander Farnsworthc,1, Philip D. Mannionb, Daniel J. Luntc, Paul J. Valdesc, Joanna V. Morgana, and Peter A. Allisona aDepartment of Earth Science and Engineering, Imperial College London, South Kensington, SW7 2AZ London, United Kingdom; bDepartment of Earth Sciences, University College London, WC1E 6BT London, United Kingdom; and cSchool of Geographical Sciences, University of Bristol, BS8 1TH Bristol, United Kingdom Edited by Nils Chr. Stenseth, University of Oslo, Oslo, Norway, and approved May 21, 2020 (received for review April 1, 2020) The Cretaceous/Paleogene mass extinction, 66 Ma, included the (17). However, the timing and size of each eruptive event are demise of non-avian dinosaurs. Intense debate has focused on the highly contentious in relation to the mass extinction event (8–10). relative roles of Deccan volcanism and the Chicxulub asteroid im- An asteroid, ∼10 km in diameter, impacted at Chicxulub, in pact as kill mechanisms for this event. Here, we combine fossil- the present-day Gulf of Mexico, 66 Ma (4, 18, 19), leaving a crater occurrence data with paleoclimate and habitat suitability models ∼180 to 200 km in diameter (Fig. 1A). This impactor struck car- to evaluate dinosaur habitability in the wake of various asteroid bonate and sulfate-rich sediments, leading to the ejection and impact and Deccan volcanism scenarios. Asteroid impact models global dispersal of large quantities of dust, ash, sulfur, and other generate a prolonged cold winter that suppresses potential global aerosols into the atmosphere (4, 18–20). These atmospheric dinosaur habitats. -

Appendix 3.Pdf
A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin Item Type Thesis or dissertation Authors Nicholls, Keith H. Citation Nicholls, K. (2019). A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin. (Doctoral dissertation). University of Chester, United Kingdom. Publisher University of Chester Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 26/09/2021 02:37:15 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/622234 International Chronostratigraphic Chart v2013/01 Erathem / Era System / Period Quaternary Neogene C e n o z o i c Paleogene Cretaceous M e s o z o i c Jurassic M e s o z o i c Jurassic Triassic Permian Carboniferous P a l Devonian e o z o i c P a l Devonian e o z o i c Silurian Ordovician s a n u a F y r Cambrian a n o i t u l o v E s ' i k s w o Ichnogeneric Diversity k p e 0 10 20 30 40 50 60 70 S 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 91 93 Number of Ichnogenera (Treatise Part W) Ichnogeneric Diversity 0 10 20 30 40 50 60 70 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 c i o 63 z 65 o e 67 a l 69 a 71 P 73 75 77 79 81 83 n 85 a i r 87 b 89 m 91 a 93 C Number of Ichnogenera (Treatise Part W) -

The Geologic Time Scale Is the Eon
Exploring Geologic Time Poster Illustrated Teacher's Guide #35-1145 Paper #35-1146 Laminated Background Geologic Time Scale Basics The history of the Earth covers a vast expanse of time, so scientists divide it into smaller sections that are associ- ated with particular events that have occurred in the past.The approximate time range of each time span is shown on the poster.The largest time span of the geologic time scale is the eon. It is an indefinitely long period of time that contains at least two eras. Geologic time is divided into two eons.The more ancient eon is called the Precambrian, and the more recent is the Phanerozoic. Each eon is subdivided into smaller spans called eras.The Precambrian eon is divided from most ancient into the Hadean era, Archean era, and Proterozoic era. See Figure 1. Precambrian Eon Proterozoic Era 2500 - 550 million years ago Archaean Era 3800 - 2500 million years ago Hadean Era 4600 - 3800 million years ago Figure 1. Eras of the Precambrian Eon Single-celled and simple multicelled organisms first developed during the Precambrian eon. There are many fos- sils from this time because the sea-dwelling creatures were trapped in sediments and preserved. The Phanerozoic eon is subdivided into three eras – the Paleozoic era, Mesozoic era, and Cenozoic era. An era is often divided into several smaller time spans called periods. For example, the Paleozoic era is divided into the Cambrian, Ordovician, Silurian, Devonian, Carboniferous,and Permian periods. Paleozoic Era Permian Period 300 - 250 million years ago Carboniferous Period 350 - 300 million years ago Devonian Period 400 - 350 million years ago Silurian Period 450 - 400 million years ago Ordovician Period 500 - 450 million years ago Cambrian Period 550 - 500 million years ago Figure 2. -

PUBLICATIONS by JAMES SPRINKLE 1965 -- Sprinkle, James
PUBLICATIONS BY JAMES SPRINKLE 1965 -- Sprinkle, James. 1965. Stratigraphy and sedimentary petrology of the lower Lodgepole Formation of southwestern Montana. M.I.T. Department of Geology and Geophysics, unpublished Senior Thesis, 29 p. (see #56 and 66 below) 1966 1. Sprinkle, James and Gutschick, R. C. 1966. Blastoids from the Sappington Formation of southwest Montana (Abst.). Geological Society of America Special Paper 87:163-164. 1967 2. Sprinkle, James and Gutschick, R. C. 1967. Costatoblastus, a channel fill blastoid from the Sappington Formation of Montana. Journal of Paleon- tology, 41(2):385-402. 1968 3. Sprinkle, James. 1968. The "arms" of Caryocrinites, a Silurian rhombiferan cystoid (Abst.). Geological Society of America Special Paper 115:210. 1969 4. Sprinkle, James. 1969. The early evolution of crinozoan and blastozoan echinoderms (Abst.). Geological Society of America Special Paper 121:287-288. 5. Robison, R. A. and Sprinkle, James. 1969. A new echinoderm from the Middle Cambrian of Utah (Abst.). Geological Society of America Abstracts with Programs, 1(5):69. 6. Robison, R. A. and Sprinkle, James. 1969. Ctenocystoidea: new class of primitive echinoderms. Science, 166(3912):1512-1514. 1970 -- Sprinkle, James. 1970. Morphology and Evolution of Blastozoan Echino- derms. Harvard University Department of Geological Sciences, unpublished Ph.D. Thesis, 433 p. (see #8 below) 1971 7. Sprinkle, James. 1971. Stratigraphic distribution of echinoderm plates in the Antelope Valley Limestone of Nevada and California. U.S. Geological Survey Professional Paper 750-D (Geological Survey Research 1971):D89-D98. 1973 8. Sprinkle, James. 1973. Morphology and Evolution of Blastozoan Echino- derms. Harvard University, Museum of Comparative Zoology Special Publication, 283 p.