“Even-Odd” Rule Encompassing Lewis's Octet Rule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CNSC Research Report 2016-17
The Science of Safety: CNSC Research Report 2016–17 © Canadian Nuclear Safety Commission (CNSC) 2018 Cat. No. CC171-24E-PDF ISSN 2369-4351 Extracts from this document may be reproduced for individual use without permission provided the source is fully acknowledged. However, reproduction in whole or in part for purposes of resale or redistribution requires prior written permission from the Canadian Nuclear Safety Commission. Également publié en français sous le titre : La science de la sûreté : Rapport de recherche de la CCSN 2016-2017 Document availability This document can be viewed on the CNSC website. To request a copy of the document in English or French, please contact: Canadian Nuclear Safety Commission 280 Slater Street P.O. Box 1046, Station B Ottawa, Ontario K1P 5S9 CANADA Tel.: 613-995-5894 or 1-800-668-5284 (in Canada only) Facsimile: 613-995-5086 Email: [email protected] Website: nuclearsafety.gc.ca Facebook: facebook.com/CanadianNuclearSafetyCommission YouTube: youtube.com/cnscccsn Twitter: @CNSC_CCSN Publishing History June 2018 Edition 1.0 Table of contents Message from the President .......................................................................................................................... 1 Introduction ................................................................................................................................................... 2 Ensuring the safety of nuclear power plants ................................................................................................. 6 Protecting -

Source of Atomic Hydrogen for Ion Trap Experiments: Review and Basic Properties
WDS'15 Proceedings of Contributed Papers — Physics, 155–161, 2015. ISBN 978-80-7378-311-2 © MATFYZPRESS Source of Atomic Hydrogen for Ion Trap Experiments: Review and Basic Properties A. Kovalenko, Š Roučka, S. Rednyk, T. D. Tran, D. Mulin, R. Plašil, J. Glosík Charles University in Prague, Faculty of Mathematics and Physics, Prague, Czech Republic. Abstract. The H-atom source was used to produce atomic hydrogen for study of ion-molecule reactions relevant to astrochemistry at low temperatures. H atoms were cooled and formed into an effusive beam, passing through a 22-pole ion trap. Here we present the basic operating principles of the H-atom source and give a review of this apparatus with some calculations of vacuum conditions and parameters of the produced H-atom beam. Introduction Hydrogen is the most abundant element in the Universe [Field et al., 1966]. It is the main component of stars, giant planets and interstellar clouds. Reactions with atomic or molecular hydrogen are important for understanding the processes which occur in the interstellar medium and during the formation of the new stars. There are three isotopes of hydrogen: protium, deuterium and tritium. The reactions of ions with H atoms have to be studied for better understanding of formation and destruction of more complex ions and molecules observed in interstellar medium. There are regions in space called H I and H II regions where hydrogen is mostly neutral in H I regions, rather than ionized or molecular in the H II regions. The atomic hydrogen plays fundamental role in many astrophysical contexts especially in formation H2 molecules [Habart et al., 2005; Sternberg et al., 2014]. -

Crystal Chemistry of Perovskite-Type Hydride Namgh3: Implications for Hydrogen Storage
Chem. Mater. 2008, 20, 2335–2342 2335 Crystal Chemistry of Perovskite-Type Hydride NaMgH3: Implications for Hydrogen Storage Hui Wu,*,†,‡ Wei Zhou,†,‡ Terrence J. Udovic,† John J. Rush,†,‡ and Taner Yildirim†,§ NIST Center for Neutron Research, National Institute of Standards and Technology, 100 Bureau DriVe, MS 6102, Gaithersburg, Maryland 20899-6102, Department of Materials Science and Engineering, UniVersity of Maryland, College Park, Maryland 20742-2115, and Department of Materials Science and Engineering, UniVersity of PennsylVania, 3231 Walnut Street, Philadelphia, PennsylVania 19104-6272 ReceiVed NoVember 26, 2007. ReVised Manuscript ReceiVed January 11, 2008 The crystal structure, lattice dynamics, and local metal-hydrogen bonding of the perovskite hydride NaMgH3 were investigated using combined neutron powder diffraction, neutron vibrational spectroscopy, and first-principles calculations. NaMgH3 crystallizes in the orthorhombic GdFeO3-type perovskite structure (Pnma) with a-b+a- octahedral tilting in the temperature range of 4 to 370 K. In contrast with previous structure studies, the refined Mg-H lengths and H-Mg-H angles indicate that the MgH6 octahedra maintain a near ideal configuration, which is corroborated by bond valence methods and our DFT calculations, and is consistent with perovskite oxides with similar tolerance factor values. The temperature dependences of the lattice distortion, octahedral tilting angle, and atomic displacement of H are also consistent with the recently observed high H mobility at elevated temperature. The stability and dynamics of NaMgH3 are discussed and rationalized in terms of the details of our observed perovskite structure. Further experiments reveal that its perovskite crystal structure and associated rapid hydrogen motion can be used to improve the slow hydrogenation kinetics of some strongly bound light-metal-hydride systems such as MgH2 and possibly to design new alloy hydrides with desirable hydrogen-storage properties. -

Highly Conductive Antiperovskites with Soft Anion Lattices 12 January 2021, by I
Highly conductive antiperovskites with soft anion lattices 12 January 2021, by I. Mindy Takamiya, Mari Toyama 'cation." They also have numerous intriguing properties, including superconductivity and, in contrast to most materials, contraction upon heating. Lithium- and sodium-rich antiperovskites, such as Li3OCl and Na3OCl, have been attracting much attention due to their high ionic conductivity and alkali metal concentration, making them promising candidates to replace liquid electrolytes used in lithium ion batteries. "But achieving a comparable lithium ion conductivity in solid materials has been challenging," explains iCeMS solid-state chemist Hiroshi Kageyama, who led the study. Soft anions, like sulfur ions (S2-), provide an ideal conduction path for sodium (Na+) and lithium (Li+) ions, Kageyama and his team synthesized a new family with the hydride ions (H-) helping to stabilize the of lithium- and sodium-rich antiperovskites that compound's structure. Credit: Mindy Takamiya/Kyoto begins to overcome this issue. Instead of 'hard' University iCeMS oxygen and halogen anions, their antiperovskites contain a hydrogen anion, called a hydride, and 'soft' chalcogen anions like sulfur. A new structural arrangement of atoms shows The scientists conducted a wide range of promise for developing safer batteries made with theoretical and experimental investigations on solid materials. Scientists at Kyoto University's these antiperovskites, and found that the soft anion Institute for Integrated Cell-Material Sciences lattice provides an ideal conduction path for lithium (iCeMS) designed a new type of 'antiperovskite' and sodium ions, which can be further enhanced by that could help efforts to replace the flammable chemical substitutions. organic electrolytes currently used in lithium ion batteries. -

Book of Abstracts
THE PHYSICS AND CHEMISTRY OF THE INTERSTELLAR MEDIUM Celebrating the first 40 years of Alexander Tielens' contribution to Science Book of Abstracts Palais des Papes - Avignon - France 2-6 September 2019 CONFERENCE PROGRAM Monday 2 September 2019 Time Speaker 10:00 Registration 13:00 Registration & Welcome Coffee 13:30 Welcome Speech C. Ceccarelli Opening Talks 13:40 PhD years H. Habing 13:55 Xander Tielens and his contributions to understanding the D. Hollenbach ISM The Dust Life Cycle 14:20 Review: The dust cycle in galaxies: from stardust to planets R. Waters and back 14:55 The properties of silicates in the interstellar medium S. Zeegers 15:10 3D map of the dust distribution towards the Orion-Eridanus S. Kh. Rezaei superbubble with Gaia DR2 15:25 Invited Talk: Understanding interstellar dust from polariza- F. Boulanger tion observations 15:50 Coffee break 16:20 Review: The life cycle of dust in galaxies M. Meixner 16:55 Dust grain size distribution across the disc of spiral galaxies M. Relano 17:10 Investigating interstellar dust in local group galaxies with G. Clayton new UV extinction curves 17:25 Invited Talk: The PROduction of Dust In GalaxIES C. Kemper (PRODIGIES) 17:50 Unravelling dust nucleation in astrophysical media using a L. Decin self-consistent, non steady-state, non-equilibrium polymer nucleation model for AGB stellar winds 19:00 Dining Cocktail Tuesday 3 September 2019 08:15 Registration PDRs 09:00 Review: The atomic to molecular hydrogen transition: a E. Roueff major step in the understanding of PDRs 09:35 Invited Talk: The Orion Bar: from ALMA images to new J. -

Ijmp.Jor.Br V
INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. 10, n. 8, Special Edition Seng 2019 ISSN: 2236-269X DOI: 10.14807/ijmp.v10i8.1046 A NEW HYPOTHESIS ABOUT THE NUCLEAR HYDROGEN STRUCTURE Relly Victoria Virgil Petrescu IFToMM, Romania E-mail: [email protected] Raffaella Aversa University of Naples, Italy E-mail: [email protected] Antonio Apicella University of Naples, Italy E-mail: [email protected] Taher M. Abu-Lebdeh North Carolina A and T State Univesity, United States E-mail: [email protected] Florian Ion Tiberiu Petrescu IFToMM, Romania E-mail: [email protected] Submission: 5/3/2019 Accept: 5/20/2019 ABSTRACT In other papers already presented on the structure and dimensions of elemental hydrogen, the elementary particle dynamics was taken into account in order to be able to determine the size of the hydrogen. This new work, one comes back with a new dynamic hypothesis designed to fundamentally change again the dynamic particle size due to the impulse influence of the particle. Until now it has been assumed that the impulse of an elementary particle is equal to the mass of the particle multiplied by its velocity, but in reality, the impulse definition is different, which is derived from the translational kinetic energy in a rapport of its velocity. This produces an additional condensation of matter in its elemental form. Keywords: Particle structure; Impulse; Condensed matter. [http://creativecommons.org/licenses/by/3.0/us/] Licensed under a Creative Commons Attribution 3.0 United States License 1749 INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. -

Laboratory Spectroscopy Techniques to Enable Observations of Interstellar Ion Chemistry
REVIEWS Laboratory spectroscopy techniques to enable observations of interstellar ion chemistry Brett A. McGuire 1,2,3 ✉ , Oskar Asvany 4, Sandra Brünken 5 and Stephan Schlemmer 4 ✉ Abstract | Molecular ions have long been considered key intermediates in the evolution of molecular complexity in the interstellar medium. However, owing to their reactivity and transient nature, ions have historically proved challenging to study in terrestrial laboratory experiments. In turn, their detection and characterization in space is often contingent upon advances in the laboratory spectroscopic techniques used to measure their spectra. In this Review, we discuss the advances over the past 50 years in laboratory methodologies for producing molecular ions and probing their rotational, vibrational and electronic spectra. + We largely focus this discussion around the widespread H3 cation and the ionic products originating from its reaction with carbon atoms. Finally, we discuss the current frontiers in this research and the technical advances required to address the spectroscopic challenges that they represent. More than 200 molecular species are now known to The primary initiator in this network is the simple + + be present in the interstellar medium (ISM), but only polyatomic cation H3 . The importance of H3 in initia- slightly more than 15% of these are ionic: either posi- ting ion–molecule chemistry has long been recognized, 1 + tively or negatively charged . Yet these charged species and H3 has been invoked as a key intermediate in have a substantial role in driving chemical evolution in reaction networks for decades2,4,5. Although laboratory space, where low densities and temperatures present knowledge of its spectrum dates back to 1980 (REF.6), considerable obstacles to reactivity. -

The Systemic Approach to Teaching and Learning
AJCE, 2020, 10(1) ISSN 2227-5835 THE “FIRST MOLECULE”: He-H+ AS A THEME TO STUDY CHEMICAL BONDING BY MOLECULAR MODELING Robson Fernandes de Farias Universidade Federal do Rio Grande do Norte, Cx. Postal 1664, 59078-970, Natal-RN, Brazil. Email: [email protected] ABSTRACT In April 2019, it was reported [3] the detection (for the first time, in the interstellar space; previous detections were in the lab) of a species that is considered the very first molecule formed in the universe: He-H+(formed about only 100,000 years after the big bang). It was the beginning of chemical synthesis, and, of course, chemistry. In the present work it is reported as such chemical species can be employed as a very good theme to study chemical bonding by using molecular modeling. [African Journal of Chemical Education—AJCE 10(1), January 2020] 1 AJCE, 2020, 10(1) ISSN 2227-5835 INTRODUCTION As previously reported [1,2] molecular modeling can be a very powerful tool to be employed as a didactical/pedagogical resource, since it allows, for example, to study chemical bonding and chemical structure in an easy and ludic approach. By molecular modeling, bond angles, lengths and energies can be “measured”, and the student can “feel” such properties and not just having to believe the data that appears in the textbooks. Of course, different theoretical approach can provide different results and to discuss such differences and to decide what approach is reliable for a given system, also enlarge so much the quality of the chemical learning. In April 2019, it was reported [3] the detection (for the first time, in the interstellar space; previous detections were in the lab) of a species that is considered the very first molecule formed in the universe: He-H+(formed about only 100,000 years after the big bang). -

Hydrogen Atomic and Molecular Hydrogen
Hydrogen Hydrogen, the simplest atom, combines with itself to make the simplest molecule, H2. Most other elements in the periodic table combine with hydrogen to make compounds. The chemical bonds between hydrogen and other elements result from sharing electrons. The electrons are shared equally in non-polar covalent bonds and unequally in polar covalent bonds. The chemical energy stored in covalent bonds can be transformed into heat or electrical energy. Outline • Atomic and Molecular Hydrogen • Hydrogen Compounds • Energy from Hydrogen • Homework Atomic and Molecular Hydrogen Electronic Structure of Atomic Hydrogen We can represent a hydrogen atom with the symbol H and a dot that stands for the single electron in the neutral atom. In its lowest energy form, the electron occupies the 1s orbital, spherically symmetrical around the hydrogen nucleus. As you saw in the section on electronic configuration, all atoms have many possible energy levels for their electrons. The lowest energy state (ground state) of an atom has its electrons filling orbitals beginning from lowest energy, with 2 electrons per orbital. When energy is added to the ground state atom, an electron can be promoted to one of the higher energy orbitals. The energy added must be exactly the same as the energy between the orbital occupied by the electron and one of the higher energy, unoccupied orbitals. Below you can see an orbital energy diagram showing the ground state hydrogen atom on the left. When hydrogen absorbs a quantity of energy exactly equal to ΔE1, the electron goes from the orbital in the first shell (n = 1) to an orbital in the second shell (n = 2). -

(12) United States Patent (10) Patent No.: US 8,333,944 B2 Constantz Et Al
USOO833394.4B2 (12) United States Patent (10) Patent No.: US 8,333,944 B2 Constantz et al. (45) Date of Patent: *Dec. 18, 2012 (54) METHODS OF SEQUESTERING CO2 61/073,319, filed on Jun. 17, 2008, provisional application No. 61/082,766, filed on Jul. 22, 2008, (75) Inventors: Brent R. Constantz, Portola Valley, CA provisional application No. 61/088,340, filed on Aug. (US); Andrew Youngs, Antelope, CA 12, 2008, provisional application No. 61/088,347, (US); Philip Brian Tuet, Milpitas, CA filed on Aug. 13, 2008, provisional application No. (US); Sidney Omelon, Willowdale (CA): 61/101,626, filed on Sep. 30, 2008, provisional Kasra Farsad, San Jose, CA (US); application No. 61/121,872, filed on Dec. 11, 2008. Ryan J. Gilliam, San Jose, CA (US); Valentin Decker, San Jose, CA (US); (51) Int. C. Donald W. Kirk, Caledon (CA); J. BOID 53/62 (2006.01) Douglas Way, Boulder, CO (US); Allen BOID 53/78 (2006.01) J. Bard, Austin, TX (US); Robert (52) U.S. C. ......... 423/225; 423/220; 423/230; 423/234 Danziger, Carmel, CA (US); Miguel (58) Field of Classification Search .................. 423/210, Fernandez, San Jose, CA (US); Cecily 423/220, 225, 230, 234 Ryan, San Jose, CA (US) See application file for complete search history. (73) Assignee: Calera Corporation, Los Gatos, CA (56) References Cited (US) U.S. PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this 1,169,766 A 2f1916 Brassert patent is extended or adjusted under 35 (Continued) U.S.C. 154(b) by 40 days. FOREIGN PATENT DOCUMENTS This patent is Subject to a terminal dis claimer. -
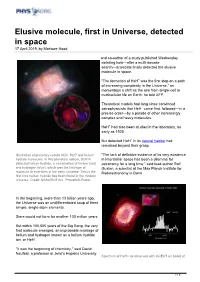
Elusive Molecule, First in Universe, Detected in Space 17 April 2019, by Marlowe Hood
Elusive molecule, first in Universe, detected in space 17 April 2019, by Marlowe Hood and co-author of a study published Wednesday detailing how—after a multi-decade search—scientists finally detected the elusive molecule in space. "The formation of HeH+ was the first step on a path of increasing complexity in the Universe," as momentous a shift as the one from single-cell to multicellular life on Earth, he told AFP. Theoretical models had long since convinced astrophysicists that HeH+ came first, followed—in a precise order—by a parade of other increasingly complex and heavy molecules. HeH+ had also been studied in the laboratory, as early as 1925. But detected HeH+ in its natural habitat had remained beyond their grasp. Illustration of planetary nebula NGC 7027 and helium "The lack of definitive evidence of its very existence hydride molecules. In this planetary nebula, SOFIA in interstellar space has been a dilemma for detected helium hydride, a combination of helium (red) astronomy for a long time," said lead author Rolf and hydrogen (blue), which was the first type of Gusten, a scientist at the Max Planck Institute for molecule to ever form in the early universe. This is the Radioastronomy in Bonn. first time helium hydride has been found in the modern universe. Credit: NASA/SOFIA/L. Proudfit/D.Rutter In the beginning, more than 13 billion years ago, the Universe was an undifferentiated soup of three simple, single-atom elements. Stars would not form for another 100 million years. But within 100,000 years of the Big Bang, the very first molecule emerged, an improbable marriage of helium and hydrogen known as a helium hydride ion, or HeH+. -

{Download PDF}
NO! PDF, EPUB, EBOOK Marta Altes | 32 pages | 15 May 2012 | Child's Play International Ltd | 9781846434174 | English | Swindon, United Kingdom trình giả lập trên PC và Mac miễn phí – Tải NoxPlayer Don't have an account? Sign up here. Already have an account? Log in here. By creating an account, you agree to the Privacy Policy and the Terms and Policies , and to receive email from Rotten Tomatoes and Fandango. Please enter your email address and we will email you a new password. We want to hear what you have to say but need to verify your account. Just leave us a message here and we will work on getting you verified. No uses its history-driven storyline to offer a bit of smart, darkly funny perspective on modern democracy and human nature. Rate this movie. Oof, that was Rotten. Meh, it passed the time. So Fresh: Absolute Must See! You're almost there! Just confirm how you got your ticket. Cinemark Coming Soon. Regal Coming Soon. By opting to have your ticket verified for this movie, you are allowing us to check the email address associated with your Rotten Tomatoes account against an email address associated with a Fandango ticket purchase for the same movie. Geoffrey Macnab. Gripping and suspenseful even though the ending is already known. Rene Rodriguez. The best movie ever made about Chilean plebiscites, No thoroughly deserves its Oscar nomination for Best Foreign Film. Anthony Lane. Soren Andersen. Calvin Wilson. A cunning and richly enjoyable combination of high-stakes drama and media satire from Chilean director Pablo Larrain.