British Chemical Abstracts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Book of Abstracts
THE PHYSICS AND CHEMISTRY OF THE INTERSTELLAR MEDIUM Celebrating the first 40 years of Alexander Tielens' contribution to Science Book of Abstracts Palais des Papes - Avignon - France 2-6 September 2019 CONFERENCE PROGRAM Monday 2 September 2019 Time Speaker 10:00 Registration 13:00 Registration & Welcome Coffee 13:30 Welcome Speech C. Ceccarelli Opening Talks 13:40 PhD years H. Habing 13:55 Xander Tielens and his contributions to understanding the D. Hollenbach ISM The Dust Life Cycle 14:20 Review: The dust cycle in galaxies: from stardust to planets R. Waters and back 14:55 The properties of silicates in the interstellar medium S. Zeegers 15:10 3D map of the dust distribution towards the Orion-Eridanus S. Kh. Rezaei superbubble with Gaia DR2 15:25 Invited Talk: Understanding interstellar dust from polariza- F. Boulanger tion observations 15:50 Coffee break 16:20 Review: The life cycle of dust in galaxies M. Meixner 16:55 Dust grain size distribution across the disc of spiral galaxies M. Relano 17:10 Investigating interstellar dust in local group galaxies with G. Clayton new UV extinction curves 17:25 Invited Talk: The PROduction of Dust In GalaxIES C. Kemper (PRODIGIES) 17:50 Unravelling dust nucleation in astrophysical media using a L. Decin self-consistent, non steady-state, non-equilibrium polymer nucleation model for AGB stellar winds 19:00 Dining Cocktail Tuesday 3 September 2019 08:15 Registration PDRs 09:00 Review: The atomic to molecular hydrogen transition: a E. Roueff major step in the understanding of PDRs 09:35 Invited Talk: The Orion Bar: from ALMA images to new J. -

Ijmp.Jor.Br V
INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. 10, n. 8, Special Edition Seng 2019 ISSN: 2236-269X DOI: 10.14807/ijmp.v10i8.1046 A NEW HYPOTHESIS ABOUT THE NUCLEAR HYDROGEN STRUCTURE Relly Victoria Virgil Petrescu IFToMM, Romania E-mail: [email protected] Raffaella Aversa University of Naples, Italy E-mail: [email protected] Antonio Apicella University of Naples, Italy E-mail: [email protected] Taher M. Abu-Lebdeh North Carolina A and T State Univesity, United States E-mail: [email protected] Florian Ion Tiberiu Petrescu IFToMM, Romania E-mail: [email protected] Submission: 5/3/2019 Accept: 5/20/2019 ABSTRACT In other papers already presented on the structure and dimensions of elemental hydrogen, the elementary particle dynamics was taken into account in order to be able to determine the size of the hydrogen. This new work, one comes back with a new dynamic hypothesis designed to fundamentally change again the dynamic particle size due to the impulse influence of the particle. Until now it has been assumed that the impulse of an elementary particle is equal to the mass of the particle multiplied by its velocity, but in reality, the impulse definition is different, which is derived from the translational kinetic energy in a rapport of its velocity. This produces an additional condensation of matter in its elemental form. Keywords: Particle structure; Impulse; Condensed matter. [http://creativecommons.org/licenses/by/3.0/us/] Licensed under a Creative Commons Attribution 3.0 United States License 1749 INDEPENDENT JOURNAL OF MANAGEMENT & PRODUCTION (IJM&P) http://www.ijmp.jor.br v. -

Laboratory Spectroscopy Techniques to Enable Observations of Interstellar Ion Chemistry
REVIEWS Laboratory spectroscopy techniques to enable observations of interstellar ion chemistry Brett A. McGuire 1,2,3 ✉ , Oskar Asvany 4, Sandra Brünken 5 and Stephan Schlemmer 4 ✉ Abstract | Molecular ions have long been considered key intermediates in the evolution of molecular complexity in the interstellar medium. However, owing to their reactivity and transient nature, ions have historically proved challenging to study in terrestrial laboratory experiments. In turn, their detection and characterization in space is often contingent upon advances in the laboratory spectroscopic techniques used to measure their spectra. In this Review, we discuss the advances over the past 50 years in laboratory methodologies for producing molecular ions and probing their rotational, vibrational and electronic spectra. + We largely focus this discussion around the widespread H3 cation and the ionic products originating from its reaction with carbon atoms. Finally, we discuss the current frontiers in this research and the technical advances required to address the spectroscopic challenges that they represent. More than 200 molecular species are now known to The primary initiator in this network is the simple + + be present in the interstellar medium (ISM), but only polyatomic cation H3 . The importance of H3 in initia- slightly more than 15% of these are ionic: either posi- ting ion–molecule chemistry has long been recognized, 1 + tively or negatively charged . Yet these charged species and H3 has been invoked as a key intermediate in have a substantial role in driving chemical evolution in reaction networks for decades2,4,5. Although laboratory space, where low densities and temperatures present knowledge of its spectrum dates back to 1980 (REF.6), considerable obstacles to reactivity. -

The Systemic Approach to Teaching and Learning
AJCE, 2020, 10(1) ISSN 2227-5835 THE “FIRST MOLECULE”: He-H+ AS A THEME TO STUDY CHEMICAL BONDING BY MOLECULAR MODELING Robson Fernandes de Farias Universidade Federal do Rio Grande do Norte, Cx. Postal 1664, 59078-970, Natal-RN, Brazil. Email: [email protected] ABSTRACT In April 2019, it was reported [3] the detection (for the first time, in the interstellar space; previous detections were in the lab) of a species that is considered the very first molecule formed in the universe: He-H+(formed about only 100,000 years after the big bang). It was the beginning of chemical synthesis, and, of course, chemistry. In the present work it is reported as such chemical species can be employed as a very good theme to study chemical bonding by using molecular modeling. [African Journal of Chemical Education—AJCE 10(1), January 2020] 1 AJCE, 2020, 10(1) ISSN 2227-5835 INTRODUCTION As previously reported [1,2] molecular modeling can be a very powerful tool to be employed as a didactical/pedagogical resource, since it allows, for example, to study chemical bonding and chemical structure in an easy and ludic approach. By molecular modeling, bond angles, lengths and energies can be “measured”, and the student can “feel” such properties and not just having to believe the data that appears in the textbooks. Of course, different theoretical approach can provide different results and to discuss such differences and to decide what approach is reliable for a given system, also enlarge so much the quality of the chemical learning. In April 2019, it was reported [3] the detection (for the first time, in the interstellar space; previous detections were in the lab) of a species that is considered the very first molecule formed in the universe: He-H+(formed about only 100,000 years after the big bang). -
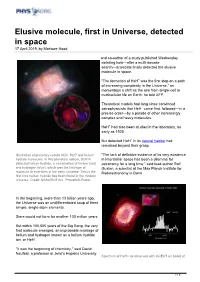
Elusive Molecule, First in Universe, Detected in Space 17 April 2019, by Marlowe Hood
Elusive molecule, first in Universe, detected in space 17 April 2019, by Marlowe Hood and co-author of a study published Wednesday detailing how—after a multi-decade search—scientists finally detected the elusive molecule in space. "The formation of HeH+ was the first step on a path of increasing complexity in the Universe," as momentous a shift as the one from single-cell to multicellular life on Earth, he told AFP. Theoretical models had long since convinced astrophysicists that HeH+ came first, followed—in a precise order—by a parade of other increasingly complex and heavy molecules. HeH+ had also been studied in the laboratory, as early as 1925. But detected HeH+ in its natural habitat had remained beyond their grasp. Illustration of planetary nebula NGC 7027 and helium "The lack of definitive evidence of its very existence hydride molecules. In this planetary nebula, SOFIA in interstellar space has been a dilemma for detected helium hydride, a combination of helium (red) astronomy for a long time," said lead author Rolf and hydrogen (blue), which was the first type of Gusten, a scientist at the Max Planck Institute for molecule to ever form in the early universe. This is the Radioastronomy in Bonn. first time helium hydride has been found in the modern universe. Credit: NASA/SOFIA/L. Proudfit/D.Rutter In the beginning, more than 13 billion years ago, the Universe was an undifferentiated soup of three simple, single-atom elements. Stars would not form for another 100 million years. But within 100,000 years of the Big Bang, the very first molecule emerged, an improbable marriage of helium and hydrogen known as a helium hydride ion, or HeH+. -

{Download PDF}
NO! PDF, EPUB, EBOOK Marta Altes | 32 pages | 15 May 2012 | Child's Play International Ltd | 9781846434174 | English | Swindon, United Kingdom trình giả lập trên PC và Mac miễn phí – Tải NoxPlayer Don't have an account? Sign up here. Already have an account? Log in here. By creating an account, you agree to the Privacy Policy and the Terms and Policies , and to receive email from Rotten Tomatoes and Fandango. Please enter your email address and we will email you a new password. We want to hear what you have to say but need to verify your account. Just leave us a message here and we will work on getting you verified. No uses its history-driven storyline to offer a bit of smart, darkly funny perspective on modern democracy and human nature. Rate this movie. Oof, that was Rotten. Meh, it passed the time. So Fresh: Absolute Must See! You're almost there! Just confirm how you got your ticket. Cinemark Coming Soon. Regal Coming Soon. By opting to have your ticket verified for this movie, you are allowing us to check the email address associated with your Rotten Tomatoes account against an email address associated with a Fandango ticket purchase for the same movie. Geoffrey Macnab. Gripping and suspenseful even though the ending is already known. Rene Rodriguez. The best movie ever made about Chilean plebiscites, No thoroughly deserves its Oscar nomination for Best Foreign Film. Anthony Lane. Soren Andersen. Calvin Wilson. A cunning and richly enjoyable combination of high-stakes drama and media satire from Chilean director Pablo Larrain. -

C Copyright 2015 Laura I. Bodine
c Copyright 2015 Laura I. Bodine Molecular Effects in Tritium Beta-Decay Neutrino-Mass Measurements Laura I. Bodine A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2015 Reading Committee: R. G. Hamish Robertson, Chair Jason Detwiler R. Jeffrey Wilkes Program Authorized to Offer Degree: Physics University of Washington Abstract Molecular Effects in Tritium Beta-Decay Neutrino-Mass Measurements Laura I. Bodine Chair of the Supervisory Committee: Professor R. G. Hamish Robertson Department of Physics An understanding of the molecular final state distribution is crucial to extracting a neutrino mass from molecular tritium-based experiments including the upcoming Karlsruhe Tritium Neutrino Mass Experiment. This dissertation studies molecular systematics for the upcoming neutrino mass measurements and discusses the mea- surement of molecular dissociation as a test of final state distribution calculations. TABLE OF CONTENTS Page List of Figures................................... iv List of Tables.................................... viii Glossary....................................... xi Chapter 1: Neutrino Mass...........................1 1.1 Introduction to Massive Neutrinos....................1 1.2 Neutrino mass in the standard model..................2 1.3 Neutrinoless double-beta decay.....................3 1.4 Astrophysics, cosmology and neutrino mass...............5 1.5 Direct neutrino mass experiments....................8 1.6 Future prospects for direct neutrino-mass experiments......... 10 Chapter 2: Molecular Tritium Decay and Neutrino Mass Measurements.. 15 2.1 Beta Decay and Neutrino Mass..................... 15 2.2 Molecular Final State Distribution................... 24 2.3 Conceptual model of the rotational-vibrational spectrum....... 32 2.4 Tests of tritium final-state calculations................. 44 Chapter 3: Tritium Recoil-Ion Mass Spectrometer.............. 63 3.1 Experimental Concept......................... -

Download Date 07/10/2021 23:40:49
OBSERVATION OF THE INFRARED SPECTRUM OF THE HELIUM-HYDRIDE MOLECULAR ION Item Type text; Dissertation-Reproduction (electronic) Authors Tolliver, David Edward Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 23:40:49 Link to Item http://hdl.handle.net/10150/282038 INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. Unless we meant to delete copyrighted materials that should not have been filmed, you will find a good image of the page in the adjacent frame. -

The Year in Chemistry 2019’S Biggest Chemistry Stories
THE YEAR IN CHEMISTRY 2019’S BIGGEST CHEMISTRY STORIES INTERNATIONAL YEAR OF THE PERIODIC TABLE EBOLA VACCINE APPROVED AND IN PRODUCTION 2019 saw celebrations to mark the 150th A new vaccine for Ebola was approved I Y anniversary of Dmitri Mendeleev’s periodic in Europe after successfully undergoing table. The year saw the oldest classroom clinical trials. A ruling on the vaccine’s US Pt periodic table uncovered, and the smallest approval is expected in March, with the and largest ever tables assembled. vaccine available from late 2020. LITHIUM-ION BATTERIES WIN NOBEL PRIZE FURTHER EVIDENCE FOR PERIODICITY BREAKDOWN The Nobel Prize in chemistry was awarded New calculations show that copernicium to the development of lithium-ion batteries Cn is a highly volatile ‘noble liquid’, and that power phones, computers, and more. Og oganesson is a metallic semiconductor. One winner, John B Goodenough, became It’s further evidence for the breakdown of the oldest ever Nobel Prize winner at 97. periodicity for the superheavy elements. A NEW RING FORM OF ELEMENTAL CARBON REDUCING & CONVERTING CO2 EMISSIONS Chemists created a new form of elemental Chemists trialled ways to reduce carbon carbon: a ring consisting solely of 18 dioxide emissions. A battery-like device carbon atoms. Meanwhile, a new algorithm can capture CO2 emissions in car exhausts, C CO2 suggests there could be 43 different forms while a copper catalyst converts CO2 from of carbon yet to be discovered. the atmosphere into useful chemicals. STIR BAR CONTAMINATION CATALYSES REACTIONS ALZHEIMER’S DRUG TRIALS UNSUCCESSFUL The magnetic stir bars used in many Clinical trials on Alzheimer’s drug laboratories are coated in an inert polymer. -

“Even-Odd” Rule Encompassing Lewis's Octet Rule
Open Journal of Physical Chemistry, 2014, 4, 67-72 Published Online May 2014 in SciRes. http://www.scirp.org/journal/ojpc http://dx.doi.org/10.4236/ojpc.2014.42010 Chemical Structural Formulas of Single-Bonded Ions Using the “Even-Odd” Rule Encompassing Lewis’s Octet Rule: Application to Position of Single-Charge and Electron-Pairs in Hypo- and Hyper-Valent Ions with Main Group Elements Geoffroy Auvert CEA-Leti, Grenoble, France Email: [email protected] Received 25 March 2014; revised 20 April 2014; accepted 28 April 2014 Copyright © 2014 by author and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Lewis developed a 2D-representation of molecules, charged or uncharged, known as structural formula, and stated the criteria to draw it. At the time, the vast majority of known molecules fol- lowed the octet-rule, one of Lewis’s criteria. The same method was however rapidly applied to re- present compounds that do not follow the octet-rule, i.e. compounds for which some of the com- posing atoms have greater or less than eight electrons in their valence shell. In a previous paper, an even-odd rule was proposed and shown to apply to both types of uncharged molecules. In the present paper, the even-odd rule is extended with the objective to encompass all single-bonded ions in one group: Lewis’s ions, hypo- and hypervalent ions. The base of the even-odd representa- tion is compatible with Lewis’s diagram. -

Noble-Gas Chemistry More Than Half a Century After the First Report of the Noble-Gas Compound
molecules Review Noble-Gas Chemistry More than Half a Century after the First Report of the Noble-Gas Compound Zoran Mazej Department of Inorganic Chemistry and Technology, Jožef Stefan Institute, Jamova cesta 39, SI–1000 Ljubljana, Slovenia; [email protected]; Tel.: +386-1-477-3301 Academic Editor: Felice Grandinetti Received: 8 June 2020; Accepted: 30 June 2020; Published: 1 July 2020 Abstract: Recent development in the synthesis and characterization of noble-gas compounds is reviewed, i.e., noble-gas chemistry reported in the last five years with emphasis on the publications issued after 2017. XeF2 is commercially available and has a wider practical application both in the laboratory use and in the industry. As a ligand it can coordinate to metal centers resulting n+ in [M(XeF2)x] salts. With strong Lewis acids, XeF2 acts as a fluoride ion donor forming [XeF]+ or [Xe F ]+ salts. Latest examples are [Xe F ][RuF ] XeF , [Xe F ][RuF ] and [Xe F ][IrF ]. 2 3 2 3 6 · 2 2 3 6 2 3 6 Adducts NgF CrOF and NgF 2CrOF (Ng = Xe, Kr) were synthesized and structurally characterized 2· 4 2· 4 at low temperatures. The geometry of XeF6 was studied in solid argon and neon matrices. + + Xenon hexafluoride is a well-known fluoride ion donor forming various [XeF5] and [Xe2F11] salts. + + A large number of crystal structures of previously known or new [XeF5] and [Xe2F11] salts were reported, i.e., [Xe2F11][SbF6], [XeF5][SbF6], [XeF5][Sb2F11], [XeF5][BF4], [XeF5][TiF5], [XeF5]5[Ti10F45], [XeF5][Ti3F13], [XeF5]2[MnF6], [XeF5][MnF5], [XeF5]4[Mn8F36], [Xe2F11]2[SnF6], [Xe2F11]2[PbF6], [XeF ] [Sn F ], [XeF ][Xe F ][CrVOF ] 2CrVIOF , [XeF ] [CrIVF ] 2CrVIOF , [Xe F ] [CrIVF ], 5 4 5 24 5 2 11 5 · 4 5 2 6 · 4 2 11 2 6 [XeF ] [CrV O F ], [XeF ] [CrV O F ] 2HF, [XeF ] [CrV O F ] 2XeOF , A[XeF ][SbF ] (A = Rb, Cs), 5 2 2 2 8 5 2 2 2 8 · 5 2 2 2 8 · 4 5 6 2 Cs[XeF5][BixSb1-xF6]2 (x = ~0.37–0.39), NO2XeF5(SbF6)2, XeF5M(SbF6)3 (M = Ni, Mg, Zn, Co, Cu, Mn and Pd) and (XeF5)3[Hg(HF)]2(SbF6)7. -
Chemistry of the Early Universe - 1 - Dr
Universe of Learning: Science Briefing 12/5/19 – Chemistry of the Early Universe - 1 - Dr. Quyen Hart 0:00 [Slide 1] Hello everyone, this is Dr. Quyen Hart from NASA's Universe of Learning. Welcome today's science briefing. Thanks to all of you for joining us and to anyone listening to the recording in the future. For this December science briefing, our panel will talk about how astronomers use novel observations to understand the formation of the first stars and of primordial molecules. Slides for today's presentation can be found on the Museum Alliance and NASA Nationwide sites, as well as NASA's Universe of Learning site. All the recordings from previous talks should be up on the websites as well. As always, if you have any issues or questions now or in the future, you can email Jeff Nee at [email protected]. Again, that's [email protected]. Or Museum Alliance members can contact him through their new team chat app and the info is on the website. Dr. David Neufeld 1:02 So, thanks everyone very much for joining us. Today we're going to be talking about a period long ago. First of all, before there were any stars, in the first briefing, and then, in the second part, we'll hear about the formation of the very first stars. Dr. David Neufeld 1:23 [Slide 2] If we could advance the slides, please. Dr. David Neufeld 1:26 [Slide 3] And one more. So, I'm David Neufeld, and I'll talk to you about what we believe was the very first type of molecule ever to form in the history of our universe.