Efficacy of Ingesting an Oral Rehydration Solution After Exercise
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Important Notice
Fraser and Neave, Limited F&N 2011 Corporate Day 26 August 2011 Important notice Certain statements in this Presentation constitute “forward-looking statements”, including forward-looking financial information. Such forward-looking statements and financial information involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of F&NL, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements and financial information. Such forward-looking statements and financial information are based on numerous assumptions regarding F&NL’s present and future business strategies and the environment in which F&NL will operate in the future. Because these statements and financial information reflect F&NL’s current views concerning future events, these statements and financial information necessarily involve risks, uncertainties and assumptions. Actual future performance could differ materially from these forward-looking statements and financial information. F&NL expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statement or financial information contained in this Presentation to reflect any change in F&NL’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statement or information is based, subject to compliance with all applicable laws and regulations and/or the rules of the SGX-ST and/or any other regulatory or supervisory body or agency. This Presentation includes market and industry data and forecast that have been obtained from internal survey, reports and studies, where appropriate, as well as market research, publicly available information and industry publications. -

2016 White Page Consulting Pvt
MOST ADMIRED BRANDS & LEADERS Volume II www.admiredbrand.com Chief Patron Prof. Malcolm McDonald Editor-in-Chief Dhruv Bhatia Chief Operating Officer Sarmad Zargar Regional Head - Asia Asif Var Director - Research M.Y. Makky Director - Corporate Communications Kriti Nagia Head - Digital Media Shahid Bhat General Manager - Strategic Alliances & Partnerships White Page International Anubha Garg Editorial Advisors White Page International is a global consulting firm with a diverse What drives their business is the zeal to make clients successful by portfolio that includes brand consulting, print advertising, creative making the most informed decisions possible. The research conducted Sadiq Zafar - India & South East Asia advertorial promotions, PR and research. The goal is to create a by White Page, is supported by R&D on the latest industry trends and Yasir Yousuf - GCC substantial and lasting improvement in the performance of its clients leading analytical techniques. The expertise lies in performing complex and strengthening their brand value. We serve to accomplish the studies analyzing data and interpreting results leading to most goals of the brand for their products and services in the form business informed decisions through tools like Brand & Consumer Research- Client Servicing journalism and PR. With more and more brands turning to marketing Industry Research- Trade Researches Case Studies & White Papers. Indian Sub-Continent: Prasoon Jha, Danish Malik content, we leverage paid channels like advertorials and social media Southeast Asia: Taye Bayewood, Charlie Zeng, Sonia Khat- coverage for optimum coverage. • Brand and Consumer Research: wani White Page aims to be the world’s leading consulting firm, this is what Provide strategic insight into market entry strategy development, GCC Nations: Muneeb Hussain, Abdullah Al Zaim drives its focus and efforts to be the ideal partner for its clients. -

Crt Color Analyzer
CRT COLOR ANALYZER CRT CRT COLOR ANALYZER CA-100Plus Application Chromaticity Adjustment, Inspection White-balance Adjustment, Inspection Cut-off Adjustment, Inspection Select the probe among the following four types. Measuring Probe (Cable length: 2m) Measuring Probe (Cable length: 5m) High luminance Measuring Probe (Cable length: 2m) High luminance Measuring Probe (Cable length: 5m) ∗Up to five probes can be connected to a single main body. Regular measuring probes and high luminance measuring probes can be connected simultaneously to a single main body. (To connect multiple probes, the optional four-point extension board (CA-B04) is necessary.) The luminance and chromaticity of display can be measured as fast as 20 times per second (maximum), FASTER reducing the time for automatic adjustment. Accuracy of ±0.002 for White,±0.004 for R,G,B ACCURATE (Chromaticity) Precise measurement can be obtained at low luminance of 0.05 cd/m2 and reducing the cycle time. 0.05 to 1000 cd/m2 (Measuring probe) LOW LUMINANCE 0.05 to 2000 cd/m2 (High luminance measuring probe) Compatible with CA-100. EASY TO USE Compact nearly A4 size (width and depth). Sample software is bundled; you can control easily by PC. Expandable up to 5 sensing probes. White balance and cutoff adjustment system CA-100-compatible mode This is PC software created using standard accessory software CA-SDK and oth- ers, and it controls the display drivers such as CA-100Plus and pattern genera- "CA-200 mode" with the CA-100Plus. In the CA-100-com- tor to measure the white or black luminance. patible mode, compatibility with the measurement data of The white and black correction coefficients are obtained from the measured lumi- CRT color analyzer CA-100 and compatibility with the nance values of the display, and they are written to the correction circuit of the RS-232C communication environment of the CA-100 are display. -

CORE MARKET BEVERAGES Malaysia BUILDING a SUSTAINABLE FUTURE
FRASER AND NEAVE, LIMITED & SUBSIDIARY COMPANIES ANNUAL REPORT 2018 CORE MARKET BEVERAGES Malaysia BUILDING A SUSTAINABLE FUTURE BEVERAGES MALAYSIA: for its soft drinks plant in Soft Drinks and No.1 Isotonic RESULTS Shah Alam, it expanded its Drink. As the first isotonic drink Despite the overall declining water plant by installing a launched in Malaysia some soft drinks market trend, 600bpm water line, adding 30 years ago, to remain FY2018 marked a turnaround a combi blow, mould and relevant to today’s consumers, for the Beverages Malaysia filling machine, and more 100PLUS continued to reinvent business. This year, Beverages than doubling the capacity itself through innovation and Malaysia revenue was 1% of its Bentong mineral water renovation. Riding on the health higher while earnings surged plant. In addition, the newly- trend amongst consumers, over twofold, thanks to installed aseptic cold-fill PET 100PLUS has been actively lower sugar cost, favourable line at the Shah Alam plant taking part in the sugar foreign currency translation would also further accelerate reduction initiative. Through and positive effect of cost Beverages Malaysia’s careful reformulation, it has control. Key category expansion into new healthier successfully reduced its sugar market share grew as the offerings in the near future. content in its entire fruity range Group stayed focused and of 100PLUS (Orange, Berry, deployed resources to drive ISOTONIC: 100PLUS Lemon Lime and the new our core categories. Isotonic F&N’s flagship brand, Blackcurrant) and earned the and carbonated soft drinks 100PLUS, continued its reign HCS awarded by the Malaysia categories grew in part by the as Malaysia’s No.1 Carbonated Ministry of Health. -

BUILDING a SUSTAINABLE FUTURE 2019 SUSTAINABILITY REPORT Contents
BUILDING A SUSTAINABLE FUTURE 2019 SUSTAINABILITY REPORT Contents Sustainability at F&N Delivering Value Through Innovation, Managing Impacts and Conducting 02 Sustainability Highlights 2019 Human Capital Development & Social Business in a Responsible Manner 03 Board Statement Collaboration 50 Eco-efficiency 04 Message from Chairman of SDC 26 Driving Economic Value 52 Water Stewardship 06 About This Report 27 Economic Performance 54 Effluents and Waste 08 About F&N 28 Innovation 58 Packaging 10 F&N Business Model 34 Empowering Our People 60 Energy and Climate Change 12 Our Supply Chain 34 Talent Management 66 Responsible Supply Chain 13 Our Sustainability Approach 42 Market Presence 66 Sustainable Sourcing 23 Our Commitment 43 Enhancing Social Well-being 71 Safety and Well-being 43 Creating Value for Society 71 Occupational Health and Safety 77 Consumer Health and Safety 82 Product and Service Labelling Appendix 84 Performance Summary 92 External Assurance Statement 94 GRI Standard Content Index 1 In line with the Group’s efforts towards greater environmental conservation, we have elected for an electronic transmission of our Annual Report and Sustainability Report. The electronic version of this Annual Report is available on Fraser and Neave, Limited’s website (fraserandneave.com/investor-relations/annual-reports). Shareholders and other interested parties who wish to receive a printed copy may order it through the website (fraserandneave.com/contact-us/request-annual-report), e-mail ([email protected]) or telephone ((65) 6318 9393). Our FY2019 Sustainability Report is only available in electronic version, and can be doenloaded at fraserandneave.com/investor-relations/corporate-sustainability. 2 Unless specifically stated otherwise, all figures in this Annual Report are quoted in Singapore Dollars. -

Annual Report 2020
OYOSHIPower .of 100PLUS. Our F&N ICE MOUNTAIN. F&NENDURING FRUIT TREE FRESH. F&N CARNATION. F&NBRANDS NUTRISOY. DAY. TIMES. F&N SEASONS. TIMES. TEAPOT. F&N SPARKLING TEAPOT. EST COLA. F&N NUTRISOY. TEAPOT. OYOSHI. F&N MAGNOLIA. TEAPOT. SUNKIST. DAY DAY. COCO LIFE. MAGNOLIA. TIMES. FARMHOUSE.annual report 100PLUS. CARNATION. TEAPOT. SUNKIST. SUNKIST. GOLD COIN. DAY. RANGER. EST COLA. 20TIMES. OYOSHI. GOLD F&N ICE MOUNTAIN.20 FRUIT TREE FRESH. MARSHALL MAGNOLIA. GOLD COIN. DAY OYOSHI. MARSHALL CAVENDISH. DRINKS. TIMES. M F&N ALIVE. CHA. DAY F&N ALIVE. DRINKS. COCO LIFE. CARNATION. CHANG. SUNKIST. DAY MAGNOLIA. ALIVE. F&N SPARKLING DRINKS. OYOSHI. DAY DAY. TEAPOT. F&N NUTRISOY. VISION To be a stable and sustainable Food & Beverage leader in the ASEAN region. MISSION To be ASEAN’s leading owner and provider of quality and innovative products that consumers choose and trust. To support our mission, we are guided firmly by our commitment to create value for our stakeholders by ensuring that our corporate actions positively impact the socio-economic and environmental factors. ABOUT US Established in 1883, F&N is a leading Southeast Asian consumer group with expertise and prominent standing in the Food & Beverage and Publishing & Printing industries. Leveraging its strengths in marketing and distribution, research and development, brands and financial management, F&N provides key resources and sets strategic directions for its subsidiary companies across both industries. Listed on the Singapore Stock Exchange, F&N ranks as one of the most established and successful companies in the region with an impressive array of renowned brands that enjoy strong market leadership. -

Crt Color Analyzer
CRT COLOR ANALYZER CRT CRT COLOR ANALYZER CA-100Plus Application Chromaticity Adjustment, Inspection White-balance Adjustment, Inspection Cut-off Adjustment, Inspection Select the probe among the following four types. Measuring Probe (Cable length: 2 m) Measuring Probe (Cable length: 5 m) High luminance Measuring Probe (Cable length: 2 m) High luminance Measuring Probe (Cable length: 5 m) ∗Up to five probes can be connected to a single main body. Regular measuring probes and high luminance measuring probes can be connected simultaneously to a single main body. (To connect multiple probes, the optional four-point extension board (CA-B04) is necessary.) The luminance and chromaticity of display can be measured as fast as 20 times per second (maximum), FASTER reducing the time for automatic adjustment. Accuracy of ±0.002 for White,±0.004 for R,G,B ACCURATE (Chromaticity) Precise measurement can be obtained at low luminance of 0.05 cd/m2 and reducing the cycle time. Range of luminance for chromaticity measurement: 0.05 to 1000 cd/m2 (Measuring probe) LOW LUMINANCE 0.05 to 2000 cd/m2 (High luminance measuring probe) Compatible with CA-100. EASY TO USE Compact nearly A4 size (width and depth). Sample software is bundled; you can control easily by PC. Expandable up to 5 sensing probes. White balance and cutoff adjustment system CA-100-compatible mode This is PC software created using standard accessory software CA-SDK and You can select the "CA-100-compatible mode" and the others, and it controls the display drivers such as CA-100Plus and pattern "CA-200 mode" with the CA-100Plus. -
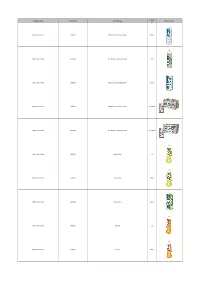
2021-04-02 School Beverage List.Xlsx
Package Company Name Brand Name Product Name Product Image Size F&N Foods Pte Ltd 100PLUS ACTIVE Non-Carbonated Drink 500ml F&N Foods Pte Ltd 100PLUS ACTIVE Non-Carbonated Drink 1.5L F&N Foods Pte Ltd 100PLUS ACTIVE Non-Carbonated Drink 300ml F&N Foods Pte Ltd 100PLUS ACTIVE Non-Carbonated Drink 6 X 300ml F&N Foods Pte Ltd 100PLUS ACTIVE Non-Carbonated Drink 12 X 300ml F&N Foods Pte Ltd 100PLUS Lemon Lime 1.5L F&N Foods Pte Ltd 100PLUS Lemon Lime 500ml F&N Foods Pte Ltd 100PLUS Lemon Lime 325ml F&N Foods Pte Ltd 100PLUS Orange 1.5L F&N Foods Pte Ltd 100PLUS Orange 500ml Package Company Name Brand Name Product Name Product Image Size F&N Foods Pte Ltd 100PLUS Original 325ml F&N Foods Pte Ltd 100PLUS Original 1.5L F&N Foods Pte Ltd 100PLUS Original 500ml F&N Foods Pte Ltd 100PLUS Original 12 X 325ml F&N Foods Pte Ltd 100PLUS Original 6 X 325ml F&N Foods Pte Ltd 100PLUS Original 12 X 1.5L F&N Foods Pte Ltd 100PLUS Original 24 X 325ml F&N Foods Pte Ltd 100PLUS Original 24 X 500ml Wanin Industries Pte Ltd 7 Select Distilled Water 1500ml Wanin Industries Pte Ltd 7 Select Distilled Water 550ml Package Company Name Brand Name Product Name Product Image Size SINHUA HOCK KEE TRADING (S) AAA 100% Natural Chrysanthemum Teabag With Brown Raw Sugar Tea 10 X 10g SINHUA HOCK KEE TRADING (S) AAA 100% Natural Ginger Powder Teabag with Brown Raw Sugar 10 X 12g McFine Marketing Pte Ltd Alamo Mineral water - R1 330ml McFine Marketing Pte Ltd Alamo Mineral water - R1 1500ml McFine Marketing Pte Ltd Alamo Mineral water - R1 600ml Allswell Trading Pte Ltd Allswell -

New Markets Overview
48 FRASER AND NEAVE, LIMITED & SUBSIDIARY COMPANIES | ANNUAL REPORT 2019 CEO Business Review New Markets Indonesia • Myanmar Thailand • Vietnam NEW IN MYANMAR • 100PLUS ACTIVE • F&N SEASONS Ready-to-Drink Tea • GOLD COIN Condensed Milk NEW IN VIETNAM • F&N Sweetened Condensed Milk in Squeeze Tube BUILDING A SUSTAINABLE FUTURE 49 NEW MARKETS lower COGs and tighter control on operating expense all contributed to improved 20 New Markets FY2019 performance across profitability from Indonesia this year. CITIES geographies were strong. Despite accelerating Our portfolio of Indonesia will remain a key new market and a competition, ongoing price discounting and products is now currency risks, each market recorded both top- core component in our growth strategy. We are available in 20 cities and bottom-line improvements. Notwithstanding determined to pursue every opportunity to grow in Indonesia ongoing challenges, Southeast Asia (“SEA”) region this important market. This will entail expanding remains the focal point of F&N’s march toward our product range, widening our reach through becoming a leading F&B player in ASEAN. expansion into other cities/regions/channels, Since 2015 when the Group outlined its Vision forging partnerships and driving consumption 2020 business plan, F&N has identified Indonesia, through localised consumer engagement Myanmar, Thailand and Vietnam as its key new activities. We will continuously review our markets. It has also identified core brands to distribution structure to ensure our ability to capitalise on clearly defined market opportunities supply products efficiently and reliably alongside and establish presence in these new markets. the rapid development of the different trade South East Asia’s population of over 640m, with channels in the region. -

Global Soft Drinks With
QUENCHING THIRST FOR OVER 80 YEARS We are known throughout the industry for our reliability, integrity and consideration for our customers’ needs. Tell us the flavour you want and we’ll make it for you. In effect, we can create or match any flavour so you can brand it up as your own. From naturals to functionals, bulk compounds or concentrates, we can meet your requirements. Even trial batches can be accommodated. Why settle for just a beverage supplier when you could have a partner? Beverage Development & Compounds Simpsons Beverage Supplyt$IJTXJDL(SPWFt#MBDLQPPMt':&6Tel: 01253 766 333 Email: [email protected] www.simpsonsbeverages.com WE MAKE THE PERFECT DRINK Soft Drinks Internationa l – December 2014/January 2015 ConTEnTS 1 news Europe 4 Africa 6 Middle East 10 Asia Pacific 14 The leading English language magazine published in Europe, devoted exclusively to the manufacture, distribution and marketing of soft drinks, fruit juices and bottled water. Americas 16 Ingredients 18 features Juices & Juice Drinks 20 Waters & Water Plus Drinks 22 Global Soft Drinks 32 After another year of growth, the global Carbonates 26 market for soft drinks now stands at a colossal 664 billion litres. However, some Sports & Energy 28 sectors have fared better than others, Functionals 29 reports Richard Corbett. RTD Teas & Coffees 30 Beer Mix And Malt Dairy & Alternatives 31 Beverages 34 In Europe, beer mix drinks are highly pop - ular, and thanks to Wild’s creative con - Processing 42 cepts, breweries and manufacturers of Packaging 45 non-alcoholic beverages can create new accents in their product palettes which Environment 48 will appeal to both traditional consumers and new target groups. -

Annual Report 2017 7 MARKET NEW PRODUCTS in FY2017 FUELLINGCAPTURINGCOST EXPORTVALUEOPTIMISATION ALONG the GROWTHSUPPLY CHAIN
F&NHBLeveragingIn transforming Group on hasthe F&NHB benefittedcombined for a strengths fromsustainable our geographical of future,our soft we drinks F&N MAGNOLIA diversityandstart dairy with overbusiness optimising the past in Malaysia; our couple resources of we years, now productivity havewhich the affirmed nation's F&N FRUIT TREE thewidestand vital cost route-to-market role effectiveness of Exports toin and thebuild widest Group's a more manufacturing growth competitive strategy. Therefore,footprintorganisation. among the Group food will& beverage be prioritising producers,OISHI on capacity which anddelivered capability greater building operational to fuel ourefficiency Exports in businessdistribution, for long-termlogisticsImprovement20 and growth. warehousing in our cost structure for the Group. and overall functionalWELL-LOVED efficiency and effectiveness from various FRASERBRANDS & NEAVE HOLDINGStransformation BHD initiatives have delivered sustainable (4205-V)financial benefits for the Group. HALAL NETWORK DECENTRALISATION F&NHB supports the development F&NHB's capital expenditure of MalaysiaCAP as a JUNJUNG global commercial® TRUSTED BY projectsand halal support hub. ourThis strategic year, the Group GENERATIONS OVER directionhas further to decentralise expanded our our export manufacturingmarket network operations. in the global We Muslimaim to optimise market where our route our halal 134 years to the extensive market that certification carried added leverage. we serve which, will provide us greater speed-to-market, cost savings -

ENCORP PACIFIC (CANADA) Registered Brands
ENCORP PACIFIC (CANADA) Registered Brands 1 7 10 Cal Aquafina Plus Vitamins 7 select 10 YEARS OIL PATCH TOUGH LONGHORN 7 Select Café 100 Plus 7 UP 100PLUS 7 up Lemon 1181 7-Select 1818 Alberni 7-SELECT 7-Up 2 7-Up 2 Guys With Knives 7D Mango Nectar 2% 7SELECT 24 Hour Collision Center 7Select 24 Mantra Mango Juice 7SELECT Natural Spring Water 24K 7UP 27 North 7up 28 Black 7up Lemon Lemon Sparkling Lemonade 3 8 3 The Terraces Three 80 Degrees 33 Acres Of Heart 80 Degrees North 33 Acres of Mineral 33 Acres of Pacific 9 33 Acres of Sunrise 9 MM Energy Drink 365 A 365 Everyday Value A & W 365 Organic Lemonade A (Futura) 365 Organic Limeade A&W 365 Whole Foods Market A&W Apple Juice 4 A&W Orange Juice 49th Parallel Cascara A-Team Mortgages 49th Parallel Cold Brew A2Z Capital 49th Parallel Grocery Abbott Wealth Management 49th Parallel Iced Tea Aberfoyle Springs 49th Parallel Sparkling Green Tea Abhishek Mehta-MarforiGroup, Mr Home Inspector ABK Restoration Services 5 Abstract Creating Iconic Spaces 5 Hour Energy Abstract Developments 5 Hour Energy Acapulcoco 5 Hour Energy Extra Strength Accelerade 5-hour Energy Extra Strength Access Roadside Assistance 5-HOUR EXTRA STRENGTH Accompass 52 North Beverages Acme Analytical Laboratories Ltd. 52° North Acqua Di Aritzia 59th Street Food Co. Acqua Filette 6 Acqua Italia 6 Hour Power Acqua Panna 601 West Hastings ACTIVATE BALANCE - Fruit Punch ACTIVATE BEAUTY - Exotic Berry ACTIVATE CHARGED - Lemon Lime Wednesday, September 01, 2021 The General Identification Guidelines should be read along with this brand registry listing.