Condensed Matter Physics Experiments List 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

“Mechanical Universe and Beyond” Videos—CE Mungan, Spring 2001
Keywords in “Mechanical Universe and Beyond” Videos—C.E. Mungan, Spring 2001 This entire series can be viewed online at http://www.learner.org/resources/series42.html. Some of the titles below have been modified by me to better reflect their contents. In my opinion, tapes 21–22 are the best in the whole series! 1. Introduction to Classical Mechanics: Kepler, Galileo, Newton 2. Falling Bodies: s = gt2 / 2, ! = gt, a = g 3. Differentiation: introductory math 4. Inertia: Newton’s first law, Copernican solar system 5. Vectors: quaternions, unit vectors, dot and cross products 6. Newton’s Laws: Newton’s second law, momentum, Newton’s third law, monkey-gun demo 7. Integration: Newton vs. Leibniz, anti-derivatives 8. Gravity: planetary orbits, universal law of gravity, the Moon falls toward the Earth 9. UCM: Ptolemaic solar system, centripetal acceleration and force 10. Fundamental Forces: Cavendish experiment, Franklin, unified theory, viscosity, tandem accelerator 11. Gravity and E&M: fundamental constants, speed of light, Oersted experiment, Maxwell 12. Millikan Experiment: CRT, scientific method 13. Energy Conservation: work, gravitational PE, KE, mechanical energy, heat, Joule, microscopic forms of energy, useful available energy 14. PE: stability, conservation, position dependence, escape speed 15. Conservation of Linear Momentum: Descartes, generalized Newton’s second law, Earth- Moon system, linear accelerator 16. SHM: amplitude-independent period of pendulum, timekeeping, restoring force, connection to UCM, elastic PE 17. Resonance: Tacoma Narrows, music, breaking wineglass demo, earthquakes, Aeolian harp, vortex shedding 18. Waves: shock waves, speed of sound, coupled oscillators, wave properties, gravity waves, isothermal vs. adiabatic bulk modulus 19. Conservation of Angular Momentum: Kepler’s second law, vortices, torque, Brahe 20. -

Caltech News
Volume 16, No.7, December 1982 CALTECH NEWS pounds, became optional and were Three Caltech offered in the winter and spring. graduate programs But under this plan, there was an overlap in material that diluted the rank number one program's efficiency, blending per in nationwide survey sons in the same classrooms whose backgrounds varied widely. Some Caltech ranked number one - students took 3B and 3C before either alone or with other institutions proceeding on to 46A and 46B, - in a recent report that judged the which focused on organic systems, scholastic quality of graduate pro" while other students went directly grams in mathematics and science at into the organic program. the nation's major research Another matter to be addressed universities. stemmed from the fact that, across Caltech led the field in geoscience, the country, the lines between inor and shared top rankings with Har ganic and organic chemistry had ' vard in physics. The Institute was in become increasingly blurred. Explains a four-way tie for first in chemistry Professor of Chemistry Peter Der with Berkeley, Harvard, and MIT. van, "We use common analytical The report was the result of a equipment. We are both molecule two-year, $500,000 study published builders in our efforts to invent new under the sponsorship of four aca materials. We use common bonds for demic groups - the American Coun The Mead Laboratory is the setting for Chemistry 5, where Carlotta Paulsen uses a rotary probing how chemical bonds are evaporator to remove a solvent from a synthesized product. Paulsen is a junior majoring in made and broken." cil of Learned Societies, the American chemistry. -

Nuclear Magnetic Resonance and Its Application in Condensed Matter Physics
Nuclear Magnetic Resonance and Its Application in Condensed Matter Physics Kangbo Hao 1. Introduction Nuclear Magnetic Resonance (NMR) is a physics phenomenon first observed by Isidor Rabi in 1938. [1] Since then, the NMR spectroscopy has been applied in a wide range of areas such as physics, chemistry, and medical examination. In this paper, I want to briefly discuss about the theory of NMR spectroscopy and its recent application in condensed matter physics. 2. Principles of NMR NMR occurs when some certain nuclei are in a static magnetic field and another oscillation magnetic field. Assuming a nucleus has a spin angular momentum 퐼⃗ = ℏ푚퐼, then its magnetic moment 휇⃗ is 휇⃗ = 훾퐼⃗ (1) The 훾 here is the gyromagnetic ratio, which depends on the property of the nucleus. If we put such a nucleus in a static magnetic field 퐵⃗⃗0, then the magnetic moment of this nuclei will process about this magnetic field. Therefore we have, [2] [3] 푑퐼⃗ 1 푑휇⃗⃗⃗ 휏⃗ = 휇⃗ × 퐵⃗⃗ = = (2) 0 푑푥 훾 푑푥 From this semiclassical picture, we can easily derive that the precession frequency 휔0 (which is called the Larmor angular frequency) is 휔0 = 훾퐵0 (3) Then, if another small oscillating magnetic field is added to the plane perpendicular to 퐵⃗⃗0, then the total magnetic field is (Assuming 퐵⃗⃗0 is in 푧̂ direction) 퐵⃗⃗ = 퐵0푧̂ + 퐵1(cos(휔푡) 푥̂ + sin(휔푡) 푦̂) (4) If we choose a frame (푥̂′, 푦̂′, 푧̂′ = 푧̂) rotating with the oscillating magnetic field, then the effective magnetic field in this frame is 휔 퐵̂ = (퐵 − ) 푧̂ + 퐵 푥̂′ (5) 푒푓푓 0 훾 1 As a result, at 휔 = 훾퐵0, which is the resonant frequency, the 푧̂ component will vanish, and thus the spin angular momentum will precess about 퐵⃗⃗1 instead. -

Giant Electron–Phonon Coupling Detected Under Surface Plasmon Resonance in Au Film
4590 Vol. 44, No. 18 / 15 September 2019 / Optics Letters Letter Giant electron–phonon coupling detected under surface plasmon resonance in Au film 1,2 3 3 1,2, FENG HE, NATHANIAL SHEEHAN, SETH R. BANK, AND YAGUO WANG * 1Department of Mechanical Engineering, The University of Texas at Austin, Austin, Texas 78712, USA 2Texas Materials Institute, The University of Texas at Austin, Austin, Texas 78712, USA 3Department of Electrical and Computer Engineering, The University of Texas at Austin, Austin, Texas 78758, USA *Corresponding author: [email protected] Received 4 July 2019; revised 22 August 2019; accepted 23 August 2019; posted 23 August 2019 (Doc. ID 371563); published 13 September 2019 Surface plasmon resonance (SPR) is a powerful tool to am- small change in reflectance/transmittance and can hardly be plify coherent phonon signals in metal films. In a 40 nm Au captured by regular coherent phonon spectroscopy. film excited with a 400 nm pump, we observed an abnor- Surface plasmon resonance (SPR) has been proven a power- mally large electron–phonon coupling constant of about ful tool to enhance the detection of CPs in metal films 17 3 8 × 10 W∕ m K, almost 40× larger than those reported [2,17–19] and dielectrics [14]. SPR is the coherent excitation in noble metals, and even comparable to transition metals. of a delocalized charge wave that propagates along an interface. We attribute this phenomenon to quantum confinement SPPs, which are the evanescent waves from the coupling be- and interband excitation. With SPR, we also observed tween light and SP in metals, can create a strongly enhanced two coherent phonon modes in a GaAs/AlAs quantum well electric field at the metal/air interface [20]. -
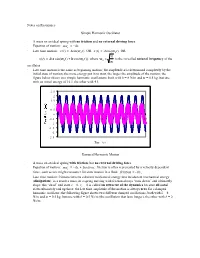
Notes on Resonance Simple Harmonic Oscillator • a Mass on An
Notes on Resonance Simple Harmonic Oscillator · A mass on an ideal spring with no friction and no external driving force · Equation of motion: max = - kx · Late time motion: x(t) = Asin(w0 t) OR x(t) = Acos(w0 t) OR k x(t) = A(a sin(w t) + b cos(w t)), where w = is the so-called natural frequency of the 0 0 0 m oscillator · Late time motion is the same as beginning motion; the amplitude A is determined completely by the initial state of motion; the more energy put in to start, the larger the amplitude of the motion; the figure below shows two simple harmonic oscillations, both with k = 8 N/m and m = 0.5 kg, but one with an initial energy of 16 J, the other with 4 J. 2.5 2 1.5 1 0.5 0 -0.5 0 5 10 15 20 -1 -1.5 -2 -2.5 Time (s) Damped Harmonic Motion · A mass on an ideal spring with friction, but no external driving force · Equation of motion: max = - kx + friction; friction is often represented by a velocity dependent force, such as one might encounter for slow motion in a fluid: friction = - bv x · Late time motion: friction converts coherent mechanical energy into incoherent mechanical energy (dissipation); as a result a mass on a spring moving with friction always “runs down” and ultimately stops; this “dead” end state x = 0, vx = 0 is called an attractor of the dynamics because all initial states ultimately end up there; the late time amplitude of the motion is always zero for a damped harmonic oscillator; the following figure shows two different damped oscillations, both with k = 8 N/m and m = 0.5 kg, but one with b = 0.5 Ns/m (the oscillation that lasts longer), the other with b = 2 Ns/m. -

AN826 Crystal Oscillator Basics and Crystal Selection for Rfpic™ And
AN826 Crystal Oscillator Basics and Crystal Selection for rfPICTM and PICmicro® Devices • What temperature stability is needed? Author: Steven Bible Microchip Technology Inc. • What temperature range will be required? • Which enclosure (holder) do you desire? INTRODUCTION • What load capacitance (CL) do you require? • What shunt capacitance (C ) do you require? Oscillators are an important component of radio fre- 0 quency (RF) and digital devices. Today, product design • Is pullability required? engineers often do not find themselves designing oscil- • What motional capacitance (C1) do you require? lators because the oscillator circuitry is provided on the • What Equivalent Series Resistance (ESR) is device. However, the circuitry is not complete. Selec- required? tion of the crystal and external capacitors have been • What drive level is required? left to the product design engineer. If the incorrect crys- To the uninitiated, these are overwhelming questions. tal and external capacitors are selected, it can lead to a What effect do these specifications have on the opera- product that does not operate properly, fails prema- tion of the oscillator? What do they mean? It becomes turely, or will not operate over the intended temperature apparent to the product design engineer that the only range. For product success it is important that the way to answer these questions is to understand how an designer understand how an oscillator operates in oscillator works. order to select the correct crystal. This Application Note will not make you into an oscilla- Selection of a crystal appears deceivingly simple. Take tor designer. It will only explain the operation of an for example the case of a microcontroller. -

Resonance Beyond Frequency-Matching
Resonance Beyond Frequency-Matching Zhenyu Wang (王振宇)1, Mingzhe Li (李明哲)1,2, & Ruifang Wang (王瑞方)1,2* 1 Department of Physics, Xiamen University, Xiamen 361005, China. 2 Institute of Theoretical Physics and Astrophysics, Xiamen University, Xiamen 361005, China. *Corresponding author. [email protected] Resonance, defined as the oscillation of a system when the temporal frequency of an external stimulus matches a natural frequency of the system, is important in both fundamental physics and applied disciplines. However, the spatial character of oscillation is not considered in the definition of resonance. In this work, we reveal the creation of spatial resonance when the stimulus matches the space pattern of a normal mode in an oscillating system. The complete resonance, which we call multidimensional resonance, is a combination of both the spatial and the conventionally defined (temporal) resonance and can be several orders of magnitude stronger than the temporal resonance alone. We further elucidate that the spin wave produced by multidimensional resonance drives considerably faster reversal of the vortex core in a magnetic nanodisk. Our findings provide insight into the nature of wave dynamics and open the door to novel applications. I. INTRODUCTION Resonance is a universal property of oscillation in both classical and quantum physics[1,2]. Resonance occurs at a wide range of scales, from subatomic particles[2,3] to astronomical objects[4]. A thorough understanding of resonance is therefore crucial for both fundamental research[4-8] and numerous related applications[9-12]. The simplest resonance system is composed of one oscillating element, for instance, a pendulum. Such a simple system features a single inherent resonance frequency. -

Understanding What Really Happens at Resonance
feature article Resonance Revealed: Understanding What Really Happens at Resonance Chris White Wood RESONANCE focus on some underlying principles and use these to construct The word has various meanings in acoustics, chemistry, vector diagrams to explain the resonance phenomenon. It thus electronics, mechanics, even astronomy. But for vibration aspires to provide a more intuitive understanding. professionals, it is the definition from the field of mechanics that is of interest, and it is usually stated thus: SYSTEM BEHAVIOR Before we move on to the why and how, let us review the what— “The condition where a system or body is subjected to an that is, what happens when a cyclic force, gradually increasing oscillating force close to its natural frequency.” from zero frequency, is applied to a vibrating system. Let us consider the shaft of some rotating machine. Rotor Yet this definition seems incomplete. It really only states the balancing is always performed to within a tolerance; there condition necessary for resonance to occur—telling us nothing will always be some degree of residual unbalance, which will of the condition itself. How does a system behave at resonance, give rise to a rotating centrifugal force. Although the residual and why? Why does the behavior change as it passes through unbalance is due to a nonsymmetrical distribution of mass resonance? Why does a system even have a natural frequency? around the center of rotation, we can think of it as an equivalent Of course, we can diagnose machinery vibration resonance “heavy spot” at some point on the rotor. problems without complete answers to these questions. -

7 Plasmonics
7 Plasmonics Highlights of this chapter: In this chapter we introduce the concept of surface plasmon polaritons (SPP). We discuss various types of SPP and explain excitation methods. Finally, di®erent recent research topics and applications related to SPP are introduced. 7.1 Introduction Long before scientists have started to investigate the optical properties of metal nanostructures, they have been used by artists to generate brilliant colors in glass artefacts and artwork, where the inclusion of gold nanoparticles of di®erent size into the glass creates a multitude of colors. Famous examples are the Lycurgus cup (Roman empire, 4th century AD), which has a green color when observing in reflecting light, while it shines in red in transmitting light conditions, and church window glasses. Figure 172: Left: Lycurgus cup, right: color windows made by Marc Chagall, St. Stephans Church in Mainz Today, the electromagnetic properties of metal{dielectric interfaces undergo a steadily increasing interest in science, dating back in the works of Gustav Mie (1908) and Rufus Ritchie (1957) on small metal particles and flat surfaces. This is further moti- vated by the development of improved nano-fabrication techniques, such as electron beam lithographie or ion beam milling, and by modern characterization techniques, such as near ¯eld microscopy. Todays applications of surface plasmonics include the utilization of metal nanostructures used as nano-antennas for optical probes in biology and chemistry, the implementation of sub-wavelength waveguides, or the development of e±cient solar cells. 208 7.2 Electro-magnetics in metals and on metal surfaces 7.2.1 Basics The interaction of metals with electro-magnetic ¯elds can be completely described within the frame of classical Maxwell equations: r ¢ D = ½ (316) r ¢ B = 0 (317) r £ E = ¡@B=@t (318) r £ H = J + @D=@t; (319) which connects the macroscopic ¯elds (dielectric displacement D, electric ¯eld E, magnetic ¯eld H and magnetic induction B) with an external charge density ½ and current density J. -

Plasmon‑Polaron Coupling in Conjugated Polymers on Infrared Metamaterials
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Plasmon‑polaron coupling in conjugated polymers on infrared metamaterials Wang, Zilong 2015 Wang, Z. (2015). Plasmon‑polaron coupling in conjugated polymers on infrared metamaterials. Doctoral thesis, Nanyang Technological University, Singapore. https://hdl.handle.net/10356/65636 https://doi.org/10.32657/10356/65636 Downloaded on 04 Oct 2021 22:08:13 SGT PLASMON-POLARON COUPLING IN CONJUGATED POLYMERS ON INFRARED METAMATERIALS WANG ZILONG SCHOOL OF PHYSICAL & MATHEMATICAL SCIENCES 2015 Plasmon-Polaron Coupling in Conjugated Polymers on Infrared Metamaterials WANG ZILONG WANG WANG ZILONG School of Physical and Mathematical Sciences A thesis submitted to the Nanyang Technological University in partial fulfilment of the requirement for the degree of Doctor of Philosophy 2015 Acknowledgements First of all, I would like to express my deepest appreciation and gratitude to my supervisor, Asst. Prof. Cesare Soci, for his support, help, guidance and patience for my research work. His passion for sciences, motivation for research and knowledge of Physics always encourage me keep learning and perusing new knowledge. As one of his first batch of graduate students, I am always thankful to have the opportunity to join with him establishing the optical spectroscopy lab and setting up experiment procedures, through which I have gained invaluable and unique experiences comparing with many other students. My special thanks to our collaborators, Professor Dr. Harald Giessen and Dr. Jun Zhao, Ms. Bettina Frank from the University of Stuttgart, Germany. Without their supports, the major idea of this thesis cannot be experimentally realized. -

NMR for Condensed Matter Physics
Concise History of NMR 1926 ‐ Pauli’s prediction of nuclear spin Gorter 1932 ‐ Detection of nuclear magnetic moment by Stern using Stern molecular beam (1943 Nobel Prize) 1936 ‐ First theoretical prediction of NMR by Gorter; attempt to detect the first NMR failed (LiF & K[Al(SO4)2]12H2O) 20K. 1938 ‐ Prof. Rabi, First detection of nuclear spin (1944 Nobel) 2015 Maglab Summer School 1942 ‐ Prof. Gorter, first published use of “NMR” ( 1967, Fritz Rabi Bloch London Prize) Nuclear Magnetic Resonance 1945 ‐ First NMR, Bloch H2O , Purcell paraffin (shared 1952 Nobel Prize) in Condensed Matter 1949 ‐ W. Knight, discovery of Knight Shift 1950 ‐ Prof. Hahn, discovery of spin echo. Purcell 1961 ‐ First commercial NMR spectrometer Varian A‐60 Arneil P. Reyes Ernst 1964 ‐ FT NMR by Ernst and Anderson (1992 Nobel Prize) NHMFL 1972 ‐ Lauterbur MRI Experiment (2003 Nobel Prize) 1980 ‐ Wuthrich 3D structure of proteins (2002 Nobel Prize) 1995 ‐ NMR at 25T (NHMFL) Lauterbur 2000 ‐ NMR at NHMFL 45T Hybrid (2 GHz NMR) Wuthrichd 2005 ‐ Pulsed field NMR >60T Concise History of NMR ‐ Old vs. New Modern Developments of NMR Magnets Technical improvements parallel developments in electronics cryogenics, superconducting magnets, digital computers. Advances in NMR Magnets 70 100T Superconducting 60 Resistive Hybrid 50 Pulse 40 Nb3Sn 30 NbTi 20 MgB2, HighTc nanotubes 10 0 1950 1960 1970 1980 1990 2000 2010 2020 2030 NMR in medical and industrial applications ¬ MRI, functional MRI ¬ non‐destructive testing ¬ dynamic information ‐ motion of molecules ¬ petroleum ‐ earth's field NMR , pore size distribution in rocks Condensed Matter ChemBio ¬ liquid chromatography, flow probes ¬ process control – petrochemical, mining, polymer production. -

Accounting for the Local Field When Determining the Dielectric Loss
materials Article Accounting for the Local Field When Determining the Dielectric Loss Spectra of Metals in the Region of the Frequencies of Volume, Surface and Localized Plasmon Oscillations Tatiana Perova 1,* , Igor Shaganov 2 and Kevin Berwick 3 1 Department of Electronic and Electrical Engineering, Trinity College Dublin, The University of Dublin, 2 Dublin, Ireland 2 Vavilov State Optical Institute, 199034 St.-Petersburg, Russia; [email protected] 3 School of Electrical and Electronic Engineering, Technical University Dublin, 8 Dublin, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +353-1-896-1432 Received: 12 December 2019; Accepted: 20 January 2020; Published: 31 January 2020 Abstract: The optical constant of bulk metal is used to determine the dispersion of the local field under one-dimensional (1D), two-dimensional (2D) and three-dimensional (3D) confinement. mic( ) 3D confinement, expressed as "2 !3D , corresponds to the dielectric loss spectra of spherical particles with a diameter, d, much less than the wavelength of the beam used to measure the spectrum (d << λ). Excellent agreement with the results of Mie theory and experimental data for solid colloids within mic( ) alkali halide crystals was observed. The function expressed as "2 !1D allows the measurement of spectral micro-characteristics in the frequency range of the longitudinal collective motion of the free electrons. This corresponds to the spectrum of dielectric losses of bulk plasma oscillations. mic( ) The function "2 !2D describes the spectra of the dielectric losses of surface plasma oscillations in mic( ) mic( ) mic( ) thin metal films. It is shown that the peak positions of "2 !3D , "2 !2D and "2 !1D spectra for simple metals, viz.