NMR for Condensed Matter Physics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Arxiv:2005.03138V2 [Cond-Mat.Quant-Gas] 23 May 2020 Contents](https://docslib.b-cdn.net/cover/8131/arxiv-2005-03138v2-cond-mat-quant-gas-23-may-2020-contents-58131.webp)
Arxiv:2005.03138V2 [Cond-Mat.Quant-Gas] 23 May 2020 Contents
Condensed Matter Physics in Time Crystals Lingzhen Guo1 and Pengfei Liang2;3 1Max Planck Institute for the Science of Light (MPL), Staudtstrasse 2, 91058 Erlangen, Germany 2Beijing Computational Science Research Center, 100193 Beijing, China 3Abdus Salam ICTP, Strada Costiera 11, I-34151 Trieste, Italy E-mail: [email protected] Abstract. Time crystals are physical systems whose time translation symmetry is spontaneously broken. Although the spontaneous breaking of continuous time- translation symmetry in static systems is proved impossible for the equilibrium state, the discrete time-translation symmetry in periodically driven (Floquet) systems is allowed to be spontaneously broken, resulting in the so-called Floquet or discrete time crystals. While most works so far searching for time crystals focus on the symmetry breaking process and the possible stabilising mechanisms, the many-body physics from the interplay of symmetry-broken states, which we call the condensed matter physics in time crystals, is not fully explored yet. This review aims to summarise the very preliminary results in this new research field with an analogous structure of condensed matter theory in solids. The whole theory is built on a hidden symmetry in time crystals, i.e., the phase space lattice symmetry, which allows us to develop the band theory, topology and strongly correlated models in phase space lattice. In the end, we outline the possible topics and directions for the future research. arXiv:2005.03138v2 [cond-mat.quant-gas] 23 May 2020 Contents 1 Brief introduction to time crystals3 1.1 Wilczek's time crystal . .3 1.2 No-go theorem . .3 1.3 Discrete time-translation symmetry breaking . -

Solid State Physics II Level 4 Semester 1 Course Content
Solid State Physics II Level 4 Semester 1 Course Content L1. Introduction to solid state physics - The free electron theory : Free levels in one dimension. L2. Free electron gas in three dimensions. L3. Electrical conductivity – Motion in magnetic field- Wiedemann-Franz law. L4. Nearly free electron model - origin of the energy band. L5. Bloch functions - Kronig Penney model. L6. Dielectrics I : Polarization in dielectrics L7 .Dielectrics II: Types of polarization - dielectric constant L8. Assessment L9. Experimental determination of dielectric constant L10. Ferroelectrics (1) : Ferroelectric crystals L11. Ferroelectrics (2): Piezoelectricity L12. Piezoelectricity Applications L1 : Solid State Physics Solid state physics is the study of rigid matter, or solids, ,through methods such as quantum mechanics, crystallography, electromagnetism and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic- scale properties. Thus, solid-state physics forms the theoretical basis of materials science. It also has direct applications, for example in the technology of transistors and semiconductors. Crystalline solids & Amorphous solids Solid materials are formed from densely-packed atoms, which interact intensely. These interactions produce : the mechanical (e.g. hardness and elasticity), thermal, electrical, magnetic and optical properties of solids. Depending on the material involved and the conditions in which it was formed , the atoms may be arranged in a regular, geometric pattern (crystalline solids, which include metals and ordinary water ice) , or irregularly (an amorphous solid such as common window glass). Crystalline solids & Amorphous solids The bulk of solid-state physics theory and research is focused on crystals. -

“Mechanical Universe and Beyond” Videos—CE Mungan, Spring 2001
Keywords in “Mechanical Universe and Beyond” Videos—C.E. Mungan, Spring 2001 This entire series can be viewed online at http://www.learner.org/resources/series42.html. Some of the titles below have been modified by me to better reflect their contents. In my opinion, tapes 21–22 are the best in the whole series! 1. Introduction to Classical Mechanics: Kepler, Galileo, Newton 2. Falling Bodies: s = gt2 / 2, ! = gt, a = g 3. Differentiation: introductory math 4. Inertia: Newton’s first law, Copernican solar system 5. Vectors: quaternions, unit vectors, dot and cross products 6. Newton’s Laws: Newton’s second law, momentum, Newton’s third law, monkey-gun demo 7. Integration: Newton vs. Leibniz, anti-derivatives 8. Gravity: planetary orbits, universal law of gravity, the Moon falls toward the Earth 9. UCM: Ptolemaic solar system, centripetal acceleration and force 10. Fundamental Forces: Cavendish experiment, Franklin, unified theory, viscosity, tandem accelerator 11. Gravity and E&M: fundamental constants, speed of light, Oersted experiment, Maxwell 12. Millikan Experiment: CRT, scientific method 13. Energy Conservation: work, gravitational PE, KE, mechanical energy, heat, Joule, microscopic forms of energy, useful available energy 14. PE: stability, conservation, position dependence, escape speed 15. Conservation of Linear Momentum: Descartes, generalized Newton’s second law, Earth- Moon system, linear accelerator 16. SHM: amplitude-independent period of pendulum, timekeeping, restoring force, connection to UCM, elastic PE 17. Resonance: Tacoma Narrows, music, breaking wineglass demo, earthquakes, Aeolian harp, vortex shedding 18. Waves: shock waves, speed of sound, coupled oscillators, wave properties, gravity waves, isothermal vs. adiabatic bulk modulus 19. Conservation of Angular Momentum: Kepler’s second law, vortices, torque, Brahe 20. -

Lecture 3: Fermi-Liquid Theory 1 General Considerations Concerning Condensed Matter
Phys 769 Selected Topics in Condensed Matter Physics Summer 2010 Lecture 3: Fermi-liquid theory Lecturer: Anthony J. Leggett TA: Bill Coish 1 General considerations concerning condensed matter (NB: Ultracold atomic gasses need separate discussion) Assume for simplicity a single atomic species. Then we have a collection of N (typically 1023) nuclei (denoted α,β,...) and (usually) ZN electrons (denoted i,j,...) interacting ∼ via a Hamiltonian Hˆ . To a first approximation, Hˆ is the nonrelativistic limit of the full Dirac Hamiltonian, namely1 ~2 ~2 1 e2 1 Hˆ = 2 2 + NR −2m ∇i − 2M ∇α 2 4πǫ r r α 0 i j Xi X Xij | − | 1 (Ze)2 1 1 Ze2 1 + . (1) 2 4πǫ0 Rα Rβ − 2 4πǫ0 ri Rα Xαβ | − | Xiα | − | For an isolated atom, the relevant energy scale is the Rydberg (R) – Z2R. In addition, there are some relativistic effects which may need to be considered. Most important is the spin-orbit interaction: µ Hˆ = B σ (v V (r )) (2) SO − c2 i · i × ∇ i Xi (µB is the Bohr magneton, vi is the velocity, and V (ri) is the electrostatic potential at 2 3 2 ri as obtained from HˆNR). In an isolated atom this term is o(α R) for H and o(Z α R) for a heavy atom (inner-shell electrons) (produces fine structure). The (electron-electron) magnetic dipole interaction is of the same order as HˆSO. The (electron-nucleus) hyperfine interaction is down relative to Hˆ by a factor µ /µ 10−3, and the nuclear dipole-dipole SO n B ∼ interaction by a factor (µ /µ )2 10−6. -

Caltech News
Volume 16, No.7, December 1982 CALTECH NEWS pounds, became optional and were Three Caltech offered in the winter and spring. graduate programs But under this plan, there was an overlap in material that diluted the rank number one program's efficiency, blending per in nationwide survey sons in the same classrooms whose backgrounds varied widely. Some Caltech ranked number one - students took 3B and 3C before either alone or with other institutions proceeding on to 46A and 46B, - in a recent report that judged the which focused on organic systems, scholastic quality of graduate pro" while other students went directly grams in mathematics and science at into the organic program. the nation's major research Another matter to be addressed universities. stemmed from the fact that, across Caltech led the field in geoscience, the country, the lines between inor and shared top rankings with Har ganic and organic chemistry had ' vard in physics. The Institute was in become increasingly blurred. Explains a four-way tie for first in chemistry Professor of Chemistry Peter Der with Berkeley, Harvard, and MIT. van, "We use common analytical The report was the result of a equipment. We are both molecule two-year, $500,000 study published builders in our efforts to invent new under the sponsorship of four aca materials. We use common bonds for demic groups - the American Coun The Mead Laboratory is the setting for Chemistry 5, where Carlotta Paulsen uses a rotary probing how chemical bonds are evaporator to remove a solvent from a synthesized product. Paulsen is a junior majoring in made and broken." cil of Learned Societies, the American chemistry. -

Nuclear Magnetic Resonance and Its Application in Condensed Matter Physics
Nuclear Magnetic Resonance and Its Application in Condensed Matter Physics Kangbo Hao 1. Introduction Nuclear Magnetic Resonance (NMR) is a physics phenomenon first observed by Isidor Rabi in 1938. [1] Since then, the NMR spectroscopy has been applied in a wide range of areas such as physics, chemistry, and medical examination. In this paper, I want to briefly discuss about the theory of NMR spectroscopy and its recent application in condensed matter physics. 2. Principles of NMR NMR occurs when some certain nuclei are in a static magnetic field and another oscillation magnetic field. Assuming a nucleus has a spin angular momentum 퐼⃗ = ℏ푚퐼, then its magnetic moment 휇⃗ is 휇⃗ = 훾퐼⃗ (1) The 훾 here is the gyromagnetic ratio, which depends on the property of the nucleus. If we put such a nucleus in a static magnetic field 퐵⃗⃗0, then the magnetic moment of this nuclei will process about this magnetic field. Therefore we have, [2] [3] 푑퐼⃗ 1 푑휇⃗⃗⃗ 휏⃗ = 휇⃗ × 퐵⃗⃗ = = (2) 0 푑푥 훾 푑푥 From this semiclassical picture, we can easily derive that the precession frequency 휔0 (which is called the Larmor angular frequency) is 휔0 = 훾퐵0 (3) Then, if another small oscillating magnetic field is added to the plane perpendicular to 퐵⃗⃗0, then the total magnetic field is (Assuming 퐵⃗⃗0 is in 푧̂ direction) 퐵⃗⃗ = 퐵0푧̂ + 퐵1(cos(휔푡) 푥̂ + sin(휔푡) 푦̂) (4) If we choose a frame (푥̂′, 푦̂′, 푧̂′ = 푧̂) rotating with the oscillating magnetic field, then the effective magnetic field in this frame is 휔 퐵̂ = (퐵 − ) 푧̂ + 퐵 푥̂′ (5) 푒푓푓 0 훾 1 As a result, at 휔 = 훾퐵0, which is the resonant frequency, the 푧̂ component will vanish, and thus the spin angular momentum will precess about 퐵⃗⃗1 instead. -

A Short Review of Phonon Physics Frijia Mortuza
International Journal of Scientific & Engineering Research Volume 11, Issue 10, October-2020 847 ISSN 2229-5518 A Short Review of Phonon Physics Frijia Mortuza Abstract— In this article the phonon physics has been summarized shortly based on different articles. As the field of phonon physics is already far ad- vanced so some salient features are shortly reviewed such as generation of phonon, uses and importance of phonon physics. Index Terms— Collective Excitation, Phonon Physics, Pseudopotential Theory, MD simulation, First principle method. —————————— —————————— 1. INTRODUCTION There is a collective excitation in periodic elastic arrangements of atoms or molecules. Melting transition crystal turns into liq- uid and it loses long range transitional order and liquid appears to be disordered from crystalline state. Collective dynamics dispersion in transition materials is mostly studied with a view to existing collective modes of motions, which include longitu- dinal and transverse modes of vibrational motions of the constituent atoms. The dispersion exhibits the existence of collective motions of atoms. This has led us to undertake the study of dynamics properties of different transitional metals. However, this collective excitation is known as phonon. In this article phonon physics is shortly reviewed. 2. GENERATION AND PROPERTIES OF PHONON Generally, over some mean positions the atoms in the crystal tries to vibrate. Even in a perfect crystal maximum amount of pho- nons are unstable. As they are unstable after some time of period they come to on the object surface and enters into a sensor. It can produce a signal and finally it leaves the target object. In other word, each atom is coupled with the neighboring atoms and makes vibration and as a result phonon can be found [1]. -
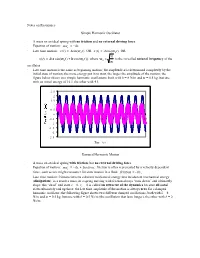
Notes on Resonance Simple Harmonic Oscillator • a Mass on An
Notes on Resonance Simple Harmonic Oscillator · A mass on an ideal spring with no friction and no external driving force · Equation of motion: max = - kx · Late time motion: x(t) = Asin(w0 t) OR x(t) = Acos(w0 t) OR k x(t) = A(a sin(w t) + b cos(w t)), where w = is the so-called natural frequency of the 0 0 0 m oscillator · Late time motion is the same as beginning motion; the amplitude A is determined completely by the initial state of motion; the more energy put in to start, the larger the amplitude of the motion; the figure below shows two simple harmonic oscillations, both with k = 8 N/m and m = 0.5 kg, but one with an initial energy of 16 J, the other with 4 J. 2.5 2 1.5 1 0.5 0 -0.5 0 5 10 15 20 -1 -1.5 -2 -2.5 Time (s) Damped Harmonic Motion · A mass on an ideal spring with friction, but no external driving force · Equation of motion: max = - kx + friction; friction is often represented by a velocity dependent force, such as one might encounter for slow motion in a fluid: friction = - bv x · Late time motion: friction converts coherent mechanical energy into incoherent mechanical energy (dissipation); as a result a mass on a spring moving with friction always “runs down” and ultimately stops; this “dead” end state x = 0, vx = 0 is called an attractor of the dynamics because all initial states ultimately end up there; the late time amplitude of the motion is always zero for a damped harmonic oscillator; the following figure shows two different damped oscillations, both with k = 8 N/m and m = 0.5 kg, but one with b = 0.5 Ns/m (the oscillation that lasts longer), the other with b = 2 Ns/m. -

Condensed Matter Physics Experiments List 1
Physics 431: Modern Physics Laboratory – Condensed Matter Physics Experiments The Oscilloscope and Function Generator Exercise. This ungraded exercise allows students to learn about oscilloscopes and function generators. Students measure digital and analog signals of different frequencies and amplitudes, explore how triggering works, and learn about the signal averaging and analysis features of digital scopes. They also explore the consequences of finite input impedance of the scope and and output impedance of the generator. List 1 Electron Charge and Boltzmann Constants from Johnson Noise and Shot Noise Mea- surements. Because electronic noise is an intrinsic characteristic of electronic components and circuits, it is related to fundamental constants and can be used to measure them. The Johnson (thermal) noise across a resistor is amplified and measured at both room temperature and liquid nitrogen temperature for a series of different resistances. The amplifier contribution to the mea- sured noise is subtracted out and the dependence of the noise voltage on the value of the resistance leads to the value of the Boltzmann constant kB. In shot noise, a series of different currents are passed through a vacuum diode and the RMS noise across a load resistor is measured at each current. Since the current is carried by electron-size charges, the shot noise measurements contain information about the magnitude of the elementary charge e. The experiment also introduces the concept of “noise figure” of an amplifier and gives students experience with a FFT signal analyzer. Hall Effect in Conductors and Semiconductors. The classical Hall effect is the basis of most sensors used in magnetic field measurements. -

Dephasing Time of Gaas Electron-Spin Qubits Coupled to a Nuclear Bath Exceeding 200 Μs
LETTERS PUBLISHED ONLINE: 12 DECEMBER 2010 | DOI: 10.1038/NPHYS1856 Dephasing time of GaAs electron-spin qubits coupled to a nuclear bath exceeding 200 µs Hendrik Bluhm1(, Sandra Foletti1(, Izhar Neder1, Mark Rudner1, Diana Mahalu2, Vladimir Umansky2 and Amir Yacoby1* Qubits, the quantum mechanical bits required for quantum the Overhauser field to the electron-spin precession before and computing, must retain their quantum states for times after the spin reversal then approximately cancel out. For longer long enough to allow the information contained in them to evolution times, the effective field acting on the electron spin be processed. In many types of electron-spin qubits, the generally changes over the precession interval. This change leads primary source of information loss is decoherence due to to an eventual loss of coherence on a timescale determined by the the interaction with nuclear spins of the host lattice. For details of the nuclear spin dynamics. electrons in gate-defined GaAs quantum dots, spin-echo Previous Hahn-echo experiments on lateral GaAs quantum dots measurements have revealed coherence times of about 1 µs have demonstrated spin-dephasing times of around 1 µs at relatively at magnetic fields below 100 mT (refs 1,2). Here, we show low magnetic fields up to 100 mT for microwave-controlled2 single- that coherence in such devices can survive much longer, and electron spins and electrically controlled1 two-electron-spin qubits. provide a detailed understanding of the measured nuclear- For optically controlled self-assembled quantum dots, coherence spin-induced decoherence. At fields above a few hundred times of 3 µs at 6 T were found5. -

AN826 Crystal Oscillator Basics and Crystal Selection for Rfpic™ And
AN826 Crystal Oscillator Basics and Crystal Selection for rfPICTM and PICmicro® Devices • What temperature stability is needed? Author: Steven Bible Microchip Technology Inc. • What temperature range will be required? • Which enclosure (holder) do you desire? INTRODUCTION • What load capacitance (CL) do you require? • What shunt capacitance (C ) do you require? Oscillators are an important component of radio fre- 0 quency (RF) and digital devices. Today, product design • Is pullability required? engineers often do not find themselves designing oscil- • What motional capacitance (C1) do you require? lators because the oscillator circuitry is provided on the • What Equivalent Series Resistance (ESR) is device. However, the circuitry is not complete. Selec- required? tion of the crystal and external capacitors have been • What drive level is required? left to the product design engineer. If the incorrect crys- To the uninitiated, these are overwhelming questions. tal and external capacitors are selected, it can lead to a What effect do these specifications have on the opera- product that does not operate properly, fails prema- tion of the oscillator? What do they mean? It becomes turely, or will not operate over the intended temperature apparent to the product design engineer that the only range. For product success it is important that the way to answer these questions is to understand how an designer understand how an oscillator operates in oscillator works. order to select the correct crystal. This Application Note will not make you into an oscilla- Selection of a crystal appears deceivingly simple. Take tor designer. It will only explain the operation of an for example the case of a microcontroller. -

Resonance Beyond Frequency-Matching
Resonance Beyond Frequency-Matching Zhenyu Wang (王振宇)1, Mingzhe Li (李明哲)1,2, & Ruifang Wang (王瑞方)1,2* 1 Department of Physics, Xiamen University, Xiamen 361005, China. 2 Institute of Theoretical Physics and Astrophysics, Xiamen University, Xiamen 361005, China. *Corresponding author. [email protected] Resonance, defined as the oscillation of a system when the temporal frequency of an external stimulus matches a natural frequency of the system, is important in both fundamental physics and applied disciplines. However, the spatial character of oscillation is not considered in the definition of resonance. In this work, we reveal the creation of spatial resonance when the stimulus matches the space pattern of a normal mode in an oscillating system. The complete resonance, which we call multidimensional resonance, is a combination of both the spatial and the conventionally defined (temporal) resonance and can be several orders of magnitude stronger than the temporal resonance alone. We further elucidate that the spin wave produced by multidimensional resonance drives considerably faster reversal of the vortex core in a magnetic nanodisk. Our findings provide insight into the nature of wave dynamics and open the door to novel applications. I. INTRODUCTION Resonance is a universal property of oscillation in both classical and quantum physics[1,2]. Resonance occurs at a wide range of scales, from subatomic particles[2,3] to astronomical objects[4]. A thorough understanding of resonance is therefore crucial for both fundamental research[4-8] and numerous related applications[9-12]. The simplest resonance system is composed of one oscillating element, for instance, a pendulum. Such a simple system features a single inherent resonance frequency.