Substance P in the Descending Cholinergic Projection to REM Sleep
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Small Dorsal Pontine Infarction Presenting with Total Gaze Palsy Including Vertical Saccades and Pursuit
Journal of Clinical Neurology / Volume 3 / December, 2007 Case Report A Small Dorsal Pontine Infarction Presenting with Total Gaze Palsy Including Vertical Saccades and Pursuit Eugene Lee, M.D., Ji Soo Kim, M.D.a, Jong Sung Kim, M.D., Ph.D., Ha Seob Song, M.D., Seung Min Kim, M.D., Sun Uk Kwon, M.D. Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine aDepartment of Neurology, Seoul National University, Bundang Hospital A small localized infarction in the dorsal pontine area can cause various eye-movement disturbances, such as abducens palsy, horizontal conjugate gaze palsy, internuclear ophthalmoplegia, and one-and-a-half syndrome. However, complete loss of vertical saccades and pursuit with horizontal gaze palsy has not been reported previously in a patient with a small pontine lesion. We report a 67-year-old man with a small dorsal caudal pontine infarct who exhibited total horizontal gaze palsy as well as loss of vertical saccades and pursuit. J Clin Neurol 3(4):208-211, 2007 Key Words : Ophthalmoplegia, Pontine infarction, Omnipause neurons A small localized dorsal pontine infarction can to admission he had experienced sudden general produce abducens palsy, horizontal conjugate gaze weakness for approximately 20 minutes without loss palsy, internuclear ophthalmoplegia (INO), and one- of consciousness while working on his farm. The and-a-half syndrome by damaging the abducens nucleus following day, the patient experienced dysarthric and its fascicle, the paramedian pontine reticular speech and visual obscuration, and his family members formation (PPRF), or the medial longitudinal fasciculus noticed that his eyes were deviated to one side. -

Micturitional Disturbance in Herpetic Brainstem Encephalitis; Contribution of the Pontine Micturition Centre
J Neurol Neurosurg Psychiatry 1998;64:269–272 269 SHORT REPORT Micturitional disturbance in herpetic brainstem encephalitis; contribution of the pontine micturition centre Ryuji Sakakibara, Takamichi Hattori, Toshio Fukutake, Masahiro Mori, Tomonori Yamanishi, Kosaku Yasuda Abstract coeruleus14 and lateral dorsal tegmental Micturitional disturbance is rarely men- nucleus.5 A pontine storage centre also exists tioned in human herpetic brainstem en- just ventromedial or lateral to the pontine mic- cephalitis although the pontine turition centre. Recently, we found micturi- tegmentum, called the pontine micturi- tional disturbance in patients with brainstem tion centre, seems to regulate the lower stroke.6 Their MRI showed that the responsible urinary tract in experimental animals. sites are comparable with those reported in The case of a 45 year old man, who devel- experimental studies.1–3 Herpes simplex virus oped subacute coma and hiccup-like dys- type 1 (HSV-1) infection also causes brainstem rhythmic breathing, and needed assisted lesions78characterised by acute onset of multi- ventilation is reported. Examination of ple cranial nerve palsies, ataxia, and pyramidal CSF showed mononuclear pleocytosis and tract involvement. Disturbances of conscious- antibody against herpes simplex virus ness and respiration are not uncommon. type 1, but the opening pressure was 90 cm Micturitional disturbance is rarely reported in this disease. We here describe the micturitional H2O. Brain CT showed brain swelling, predominantly in the posterior fossa, and disturbance of a patient with herpetic brain- bilateral subdural eVusion. Herpetic stem encephalitis who showed bilateral pontine brainstem encephalitis was diagnosed, tegmental lesions on MRI. and he received 900 mg/day vidarabine. On regaining consciousness, he had left Case report trochlear nerve palsy, left corectopia, A 45 year old, previously healthy man devel- ageusia, and urinary retention. -

Localization and Network of Coma- Causing Brainstem Lesions: Evidence for a Human Consciousness Network
Localization and Network of Coma- Causing Brainstem Lesions: Evidence for a Human Consciousness Network The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Fischer, David B. 2016. Localization and Network of Coma-Causing Brainstem Lesions: Evidence for a Human Consciousness Network. Doctoral dissertation, Harvard Medical School. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:27007725 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Abstract Focal brainstem lesions can disrupt arousal and cause coma, yet the exact location of the brainstem region critical to arousal and its associated network are unknown. First, we compare brainstem lesions between 12 patients with coma and 24 patients without coma to identify a region specific to coma-causing lesions. Second, we determine the network connectivity of this brainstem region and each individual coma- causing lesion using resting state functional connectivity MRI data acquired from 98 healthy subjects. Third, we evaluate the functional connectivity of this network in patients with disorders of consciousness (51 patients versus 21 controls). These analyses reveal a small, coma-specific region in the left pontine tegmentum, near the medial parabrachial nucleus. This brainstem region, and each individual coma-causing lesion, is functionally connected to the left agranular, anterior insula (AI), and pregenual anterior cingulate cortex (pACC). These cortical sites align poorly with previously defined functional networks but match the distribution of von Economo neurons (VENs). -

Clinicoradiological Aspects of Pontine
Published online: 2021-07-26 NEURORADIOLOGY & HEAD AND NECK IMAGING Clinicoradiological aspects of pontine tegmental cap dysplasia: Case report of a rare hindbrain malformation Aanchal Bhayana, Sunil K Bajaj, Ritu N Misra, S Senthil Kumaran1 Department of Radiodiagnosis, Safdarjung Hospital and VM Medical College, 1Department of Nuclear Medical Resonance, All India Institute of Medical Sciences, New Delhi, India Correspondence: Dr. Aanchal Bhayana, Department of Radiodiagnosis, Safdarjung Hospital and VM Medical College, New Delhi - 110 029, India. E-mail: [email protected] Abstract Malformations involving the brainstem are very rare and present with a varied spectrum of clinical symptoms due to multiple cranial nerve palsies and pyramidal tract involvement. Of these, pontine tegmental cap dysplasia is a very unusual malformation, characterized by ventral pons hypoplasia and an ectopic dorsal band of tissue, projecting into the fourth ventricle, from dorsal pontine tegmentum. A 4‑year‑old male child, presenting with left facial nerve palsy, revealed hypoplastic ventral pons and an ectopic structure on magnetic resonance imaging (MRI). The ectopic structure was isointense to pons, arose from the left side of dorsal pontine tegmentum, at pontomedullary junction and protruded into the fourth ventricle, impinging upon the left seventh and eighth cranial nerves. Diffusion tensor imaging (DTI) depicted abnormal white matter tracts in ectopic tissue with absent transverse pontine fibres and abnormal middle and superior cerebellar peduncles. -

Traumatic Transection of the Brainstem
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.44.12.1156 on 1 December 1981. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1981 ;44:1156-1158 Short report Traumatic transection of the brainstem BRIAN HARDING, MAGDA ERDOHAZI From the Department of Neuropathology, The National Hospital for Nervous Diseases, London SUMMARY A case of nearly complete transection of the lower brainstem following skull fracture with detailed histological study is presented. Brainstem lesions are a well recognised cause of parietal bones and the squamous part of the occipital coma and early death following severe head injuryl-3 bone. The main fracture line continued forwards along but massive lower brainstem laceration resulting the right temporal bone, turning medially at the base, and injury is highly unusual.45 We was seen to reach the medial end of the petrous bone. from closed head Some epidural haemorrhage was present mainly on the report here one such case which in addition had some right side, but subdural haemorrhage was much more unexpected histological features. extensive and bilateral. There were large areas of con- tusion of both frontal lobes, mainly on their orbital Case report and over both temporal poles, and this was more surfaces, Protected by copyright. An 8-year-old boy was in good health until his injury. extensive on the left side than the right. The posterior While playing on the roof of a local technical college, he temporal lobes and the lateral and inferior surfaces of the tried to jump on to a lower roof and fell through a closed occipital lobes were also contused, but here the damage glass sky-light on to the floor of a laboratory workshop was more severe on the right. -

Pontine Tegmental Cap Dysplasia: MR Imaging and Diffusion Tensor Imaging Features of ORIGINAL RESEARCH Impaired Axonal Navigation
Pontine Tegmental Cap Dysplasia: MR Imaging and Diffusion Tensor Imaging Features of ORIGINAL RESEARCH Impaired Axonal Navigation P. Jissendi-Tchofo BACKGROUND AND PURPOSE: Malformations of the brain stem are uncommon. We present MR D. Doherty imaging and diffusion tensor imaging (DTI) features of 6 patients with pontine tegmental cap dysplasia, characterized by ventral pontine hypoplasia and a dorsal “bump,” and speculate on potential mecha- G. McGillivray nisms by which it forms. R. Hevner D. Shaw MATERIALS AND METHODS: Birth and developmental records of 6 patients were reviewed. We reviewed MR imaging studies of all patients and DTIs of patient 3. Potential developmental causes G. Ishak were evaluated. R. Leventer RESULTS: All patients were born uneventfully after normal pregnancies except patient 6 (in utero A.J. Barkovich growth retardation). They presented with multiple cranial neuropathies and evidence of cerebellar dysfunction. Variable hypotonia and motor dysfunction were present. Imaging revealed ventral pontine hypoplasia and mild cerebellar vermian hypoplasia, in addition to an unusual rounded to beaklike “bump” on the dorsal surface of the pons, extending into the fourth ventricle. Color fractional anisotropy maps showed the bump to consist of a bundle of axons directed horizontally (left-right). The bump appeared, on morphologic images, to be continuous with the middle cerebellar peduncles (MCPs), which were slightly diminished in size compared with those in healthy infants. Analysis of the DTI was, however, inconclusive regarding the connections of these axons. The decussation of the MCPs, transverse pontine fibers, and longitudinal brain stem axonal pathways was also abnormal. CONCLUSIONS: Our data suggest that the dorsal transverse axonal band in these disorders results from abnormal axonal pathfinding, abnormal neuronal migration, or a combination of the 2 processes. -

An Anatomic, Imaging, and Clinical Review of the Medial Longitudinal
www.clinicalimagingscience.org Journal of Clinical Imaging Science Neuroradiology/Head and Neck Imaging Review Article An Anatomic, Imaging, and Clinical Review of the Medial Longitudinal Fasciculus Peter Fiester1, Saif Ahmed Baig1, Jeet Patel1, Dinesh Rao1 1Department of Neuroradiology, University of Florida Health Jacksonville, Jacksonville, Florida, United States. ABSTRACT e medial longitudinal fasciculus (MLF) is a paired, highly specialized, and heavily myelinated nerve bundle responsible for extraocular muscle movements, including the oculomotor reflex, saccadic eye movements an smooth pursuit, and the vestibular ocular reflex. Clinically, lesions of the MLF are classically associated with internuclear ophthalmoplegia. However, clinical manifestations of a lesion in the MLF may be more complex and variable. We provide an overview of the neuroanatomy, neurologic manifestations, and correlative examples of the *Corresponding author: imaging findings on brain MRI of MLF lesions to provide the clinician and radiologist with a more comprehensive Saif Ahmed Baig, understanding of the MLF and potential clinical manifestations for an MLF lesion. Department of Neuroradiology, University of Florida Health Keywords: Medial longitudinal fasciculus, Internuclear opthalmoplegia, Trochlear syndrome, One-and-a-half Jacksonville, Jacksonville, syndrome, Wall-eyed bilateral internuclear opthalmoparesis syndrome Florida, United States. [email protected] INTRODUCTION Received : 07 April 2020 Internuclear opthalmoplegia is defined as the lack of adduction of the ipsilateral eye with Accepted : 10 November 2020 preserved abduction of the contralateral eye with nystagmus. It is the result of a lesion Published : 18 December 2020 affecting the medial longitudinal fasciculus (MLF) – a paired, highly specialized, and heavily DOI: myelinated nerve bundle traveling in a craniocaudid direction near the midline within 10.25259/JCIS_49_2020 the tegmentum of the midbrain and dorsal pons. -

Premonitory Symptoms of Stroke in Evolution to the Locked-In State
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.46.3.221 on 1 March 1983. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1983 ;46:221-226 Premonitory symptoms of stroke in evolution to the locked-in state JOHN LIU, STANLEY TUHRIM,* JESSE WEINBERGER,* SUN K. SONG,t PAUL J ANDERSONt From the Department ofNeurology* and Division ofNeuropathologyt The Mount Sinai School ofMedicine, City University ofNew York, and the Division ofNeuropathology, City Hospital Centre at Elmhurstt, New York, USA SUMMARY Three patients, who subsequently developed the locked-in state characterised by quadriplegia and mutism with an alert sensorium, initially had mild dysarthria and uncrossed hemisensory or hemimotor deficits involving the face and ipsilateral extremities. Case one ini- tially mimicked a left cerebral lesion with right hemisensory deficits, a mild right facial paresis and a right homonymous field deficit. Case two initially developed both left hemimotor and hemisen- sory deficits and later developed a paresis of right conjugate gaze. Case three presented with left hemimotor deficit, and mild paresis of conjugate gaze to the right. All three patients died. Rostral were brainstem infarctions found at necropsy in cases one and two. Case three had a radiolucent Protected by copyright. area of the brainstem demonstrated by CT Scan. Hemisensory and hemimotor deficits also have been noted to precede reported cases of pontine infarction with the locked-in state. Acute onset of uncrossed hemisensory and hemimotor deficits with dysarthria may be caused by infarction of the pons which may predispose to the locked-in state. The locked-in state is a devastating neurological ler infarction in the midventral pons was found. -

Lab 3. Pons & Midbrain
Lab 3. Pons & Midbrain Lesion Lessons Lesion 4.1 Anne T. Pasta i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; E.C. Alvord, Jr., Univ. of Washington) iii) Cause: Lesion 4.2 Colin S. Terase i) Location ii) Signs/symptoms (Slice of Brain © 993 Univs. of Utah and Washington; M.Z. Jones, Michigan St. Univ.) iii) Cause: Medical Neuroscience 4– Pontine Level of the Facial Genu Locate and note the following: Basilar pons – massive ventral structure provides the most obvious change from previous med- ullary levels. Question classic • pontine gray - large nuclear groups in the basilar pons. Is the middle cerebellar peduncle composed – origin of the middle cerebellar peduncle of climbing or mossy • pontocerebellar axons - originate from pontine gray neurons and cross to form the fibers? middle cerebellar peduncle. • corticopontine axons- huge projection that terminates in the basilar pontine gray. • corticospinal tract axons – large bundles of axons surrounded by the basilar pontine gray. – course caudally to form the pyramids in the medulla. Pontine tegmentum • medial lemniscus - has now assumed a “horizontal” position and forms part of the border between the basilar pons and pontine tegmentum. Question classic • central tegmental tract - located just dorsally to the medial lemniscus. What sensory modali- – descends from the midbrain to the inferior olive. ties are carried by the • superior olivary nucleus - pale staining area lateral to the central tegmental tract. medial and lateral – gives rise to the efferent olivocochlear projection to the inner ear. lemnisci? • lateral lemniscus - lateral to the medial lemniscus. – composed of secondary auditory projections from the cochlear nuclei. -

Subjective of Brain Stem
Brain Disorders & Therapy Editorial Subjective of Brain Stem Samy McFarlane* Department of Medicine and Endocrinology, Osaka University, New York, USA EDOTRIAL NOTE ON BRAIN STEM METENCEPHALON The midbrain (mesencephalon) contains the atomic complex of The pons (metencephalon) comprises of two sections: the the oculomotor nerve just as the trochlear core; these cranial tegmentum, a phylogenetically more seasoned part that contains nerves innervate muscles that move the eye and control the state the reticular development, and the pontine cores, a bigger part of the focal point and the breadth of the student. Also, between made out of masses of neurons that lie among enormous heaps the midbrain reticular arrangement (referred to here as the of longitudinal and cross over nerve filaments. Filaments tegmentum) and the crus cerebri is a huge pigmented core called beginning from neurons in the cerebral cortex end upon the the substantia nigra. The substantia nigra comprises of two pontine cores, which thusly task to the contrary side of the sections, the standards reticulata and the standards compacta. equator of the cerebellum. These gigantic crossed strands, called Cells of the standards compacta contain the dim shade melanin; crus cerebri, structure the center cerebellar peduncle and fill in these cells integrate dopamine and undertaking to either the as the scaffold that associates each cerebral side of the equator caudate core or the putamen. By restraining the activity of huge with the contrary portion of the cerebellum. The filaments aspiny striatal neurons in the caudate core and the putamen beginning from the cerebral cortex establish the corticopontine (depicted above in the segment Basal ganglia), the dopaminergic lot. -

A Review of Facial Nerve Anatomy
A Review of Facial Nerve Anatomy Terence M. Myckatyn, M.D.1 and Susan E. Mackinnon, M.D.1 ABSTRACT An intimate knowledge of facial nerve anatomy is critical to avoid its inadvertent injury during rhytidectomy, parotidectomy, maxillofacial fracture reduction, and almost any surgery of the head and neck. Injury to the frontal and marginal mandibular branches of the facial nerve in particular can lead to obvious clinical deficits, and areas where these nerves are particularly susceptible to injury have been designated danger zones by previous authors. Assessment of facial nerve function is not limited to its extratemporal anatomy, however, as many clinical deficits originate within its intratemporal and intracranial components. Similarly, the facial nerve cannot be considered an exclusively motor nerve given its contributions to taste, auricular sensation, sympathetic input to the middle meningeal artery, and parasympathetic innervation to the lacrimal, submandibular, and sublingual glands. The constellation of deficits resulting from facial nerve injury is correlated with its complex anatomy to help establish the level of injury, predict recovery, and guide surgical management. KEYWORDS: Extratemporal, intratemporal, facial nerve, frontal nerve, marginal mandibular nerve The anatomy of the facial nerve is among the components of the facial nerve reminds the surgeon that most complex of the cranial nerves. In his initial descrip- the facial nerve is composed not exclusively of voluntary tion of the cranial nerves, Galen described the facial motor fibers but also of parasympathetics to the lacrimal, nerve as part of a distinct facial-vestibulocochlear nerve submandibular, and sublingual glands; sensory innerva- complex.1,2 Although the anatomy of the other cranial tion to part of the external ear; and contributions to taste nerves was accurately described shortly after Galen’s at the anterior two thirds of the tongue. -
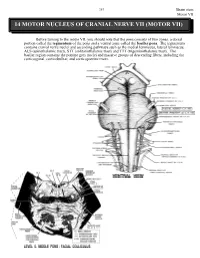
14 Motor Nucleus of Cranial Nerve Vii (Motor Vii)
263 Brain stem Motor VII 14 MOTOR NUCLEUS OF CRANIAL NERVE VII (MOTOR VII) Before turning to the motor VII, you should note that the pons consists of two zones, a dorsal portion called the tegmentum of the pons and a ventral zone called the basilar pons. The tegmentum contains cranial nerve nuclei and ascending pathways such as the medial lemniscus, lateral lemniscus, ALS (spinothalamic tract), STT (solitariothalamic tract) and TTT (trigeminothalamic tract). The basilar region contains the pontine grey nuclei and massive groups of descending fibers, including the corticospinal, corticobulbar, and corticopontine tracts. Brain stem 264 Motor VII The motor nucleus VII contains motor neurons (branchiomotor) that innervate the muscles of facial expression including the orbicularis oculi (CLOSES eyelid), the stapedius, the stylohyoid and the posterior belly of the digastric. Neurons comprising motor VII possess axons that pursue a rather circuitous route in order to exit the brain stem. Initially they pass dorsally and medially to loop over the abducens nucleus. The fibers then course ventrally and laterally to exit the brain stem. The bump in the floor of the fourth ventricle caused by the motor fibers of C.N. VII looping over the abducens nucleus is called the FACIAL COLLICULUS. A unilateral lesion interrupting the axons of C.N. VII results in the following: On the ipsilateral side, the forehead is immobile, the corner of the mouth sags, the nasolabial folds of the face are flattened, facial lines are lost, and saliva may drip from the corner of the mouth. The patient is unable to whistle or puff the cheek because the buccinator muscle is paralyzed.